Fig. 4.
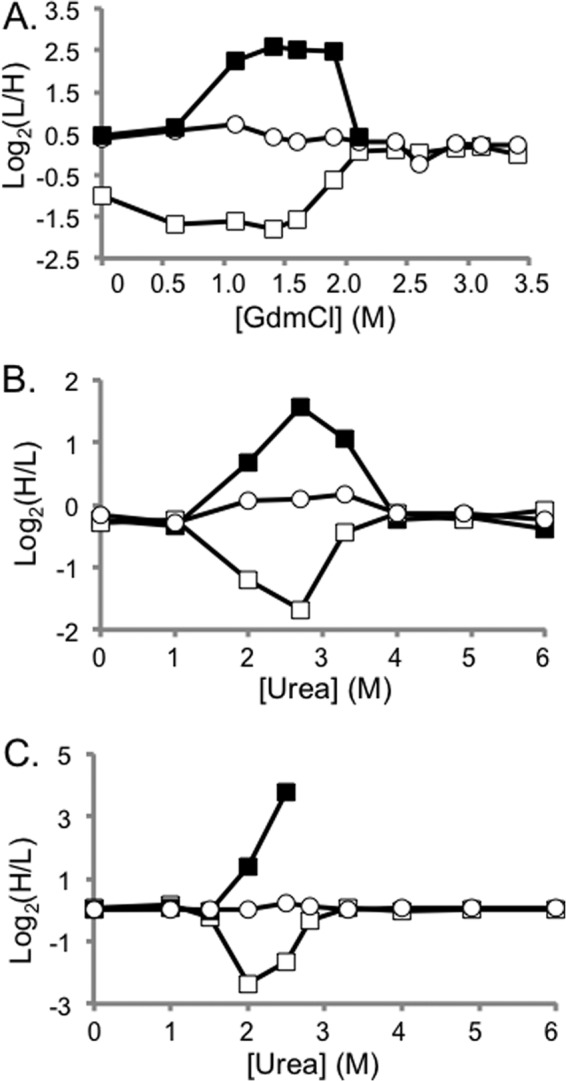
Representative SILAC-SPROX data obtained on protein hits identified in the CsA- and ATP-binding experiments using the solution-based approach. Shown in A, are CsA-binding results from Replicate 2 that were obtained on CypA peptides including the +3 charge state of the nonmethionine containing peptide HVVFGEVVDGYDIVK (open circles) and the oxidized and nonoxidized versions (albeit the +2 and +3 charge states, respectively) of a methionine-containing peptide VIPDFMLQGGDFTAGNGTGGK (open and closed squares, respectively). Shown in B, are ATP-binding results from Solution Experiment 1B that were obtained on PGM-1 peptides including a nonmethionine containing peptide, LSRAIQTANIALEK (open circles) and the oxidized and nonoxidized version of a methionine-containing peptide TVMIAAHGNSLRGLVK (open and closed squares, respectively). Shown in C, are the ATP-binding results from Solution Experiment 2 that were obtained on the same PGM-1 peptides as in B. In all cases the methionine-containing peptide data are consistent with that expected for ligand induced stabilizations. A comparison of the data in B, and C, shows that the more aggressive reaction conditions and increased ligand concentration produced more dramatically altered H/L ratios.
