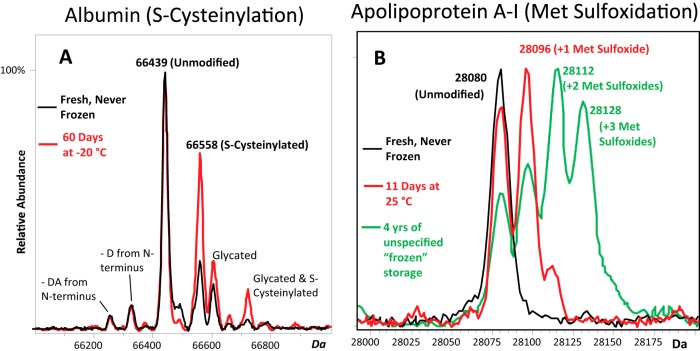
Fig. 1.
Charge deconvoluted electrospray ionization mass spectra of (A) albumin and (B) apoA-I from healthy donors showing increasing S-cysteinylated albumin and methionine-oxidized apoA-I under less-than-ideal storage conditions. Red and black spectra are from the same individual sample, aged as indicated. As evidenced, albumin is subject to minor N-terminal truncations as well as glycation (i.e. covalent glucose attachment to protein amino groups akin to the diabetes marker HbA1c). There are three methionine residues in apoA-I, permitting up to three sulfoxidation events, each of which shifts the mass of the protein up by 16 Da. The heavily oxidized apoA-I sample from a healthy donor (green spectrum) was obtained from a for-profit biobank after four years of storage under unspecified “frozen” storage conditions. (A lot-paired sample from a different healthy individual was similarly oxidized; not shown.)