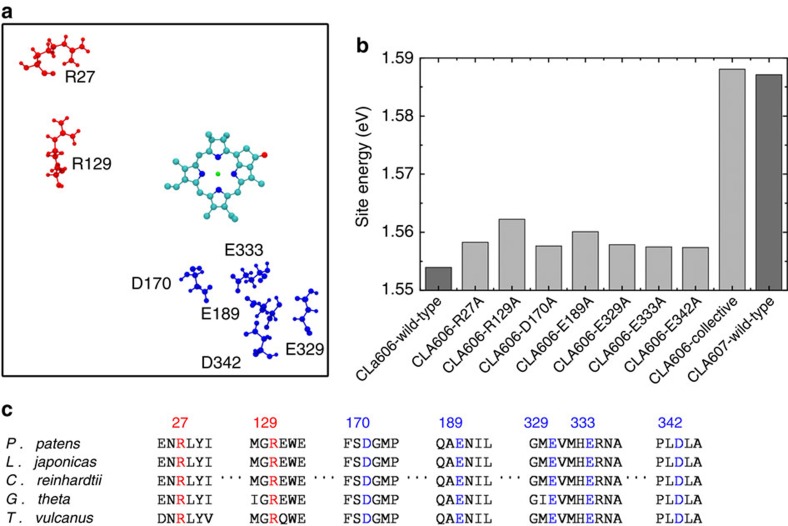
Figure 6. Several conserved residues collectively reduce the site energy of CLA606.
(a) Charged residues (red: positive-charged; blue: negative-charged) can reduce the site energy of CLA606 over 0.0035, eV individually. The tail of CLA606 is omitted in the figure for clarity. (b) Site energies of CLA606 on seven single mutations are shown. The collective mutations of all these seven residues dramatically increase CLA606’s site energy to almost the same value as its counterpart cofactor (CLA607) in the inactive chain. The wild-type site energies for both CLA606 and CLA607 are also displayed for reference. (c) These seven residues are highly conserved among different species. The sequence alignment was performed with the ClustalW2 program68,69.