Figure 1.
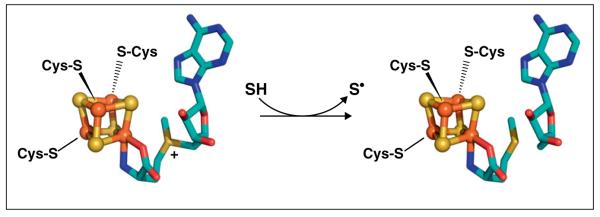
The reductive cleavage of SAM catalyzed by RS enzymes. SAM is coordinated via its amino and carboxyl moieties to the unique iron of a [4Fe–4S] cluster (left). The reduced [4Fe–4S]+ cluster transfers an electron to SAM, thereby promoting homolytic cleavage to generate methionine and a 5′-deoxyadenosyl radical. The 5′-deoxyadenosyl radical abstracts a hydrogen atom from substrate (SH) to produce a substrate radical (S•), methionine, and 5′-deoxyadenosine (right).
