Figure 1.
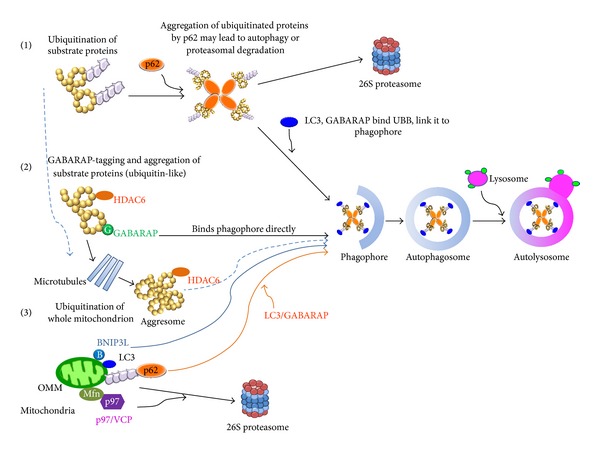
Diagram of candidate pathways leading to sperm mitophagy by autophagy and ubiquitin-proteasome system. Generally, the process of autophagy starts with the aggregation and ubiquitination of proteins or organelles that need to be recycled. Multiubiquitin chains on such aggregates are recognized by the ubiquitin-binding autophagy receptors and are brought to phagophore, a membranous organelle that eventually closes around the protein aggregate to form an autophagosome. In the finals step, autophagosome fuses with a lysosome that contains proteases able to degrade the protein cargo. In some branches of this pathway, protein aggregates or ubiquitinated proteins extracted from organelle membranes are targeted for degradation by the 26S proteasome, a multisubunit ubiquitin-specific protease. At least three previously characterized pathways could be involved in the degradation of sperm mitochondria inside a fertilized oocyte: (1) Autophagy-associated ubiquitin-receptor p62/SQSTM1 recognizes ubiquitinated cargo and interacts with autophagosome-binding ubiquitin-like proteins, such as LC3 or GABARAP; these autophagy receptors guide the protein cargo to phagophore; (2) ubiquitinated proteins of mitochondrial origin form aggresomes, the protein aggregates induced by the ubiquitin-binding adaptor protein HDAC6, which transport the ubiquitinated proteins towards degradation site, the phagophore, along microtubule tracks. (3) Protein dislocase p97/VCP extracts and presents the ubiquitinated mitochondrial membrane proteins to the 26S proteasome, the ubiquitin-dependent protease, without the involvement of phagophore.
