Abstract
Although diffusion tensor imaging (DTI) studies have reported fractional anisotropy (FA) abnormalities in multiple white matter (WM) regions in schizophrenia, relationship between abnormal FA and negative symptoms has not been fully explored. DTI data were acquired from twenty-four patients with chronic schizophrenia and twenty-five healthy controls. Regional brain abnormalities were evaluated by conducting FA comparisons in the cerebral and each lobar WMs between groups. Focal abnormalities were also evaluated with a voxel-wise tract specific method. Associations between structural WM changes and negative symptoms were assessed using the Scale for the Assessment of Negative Symptoms (SANS). The patient group showed decreased FA in the cerebrum, especially in the frontal lobe, compared with controls. A voxel-wise analysis showed FA decreases in almost all WM tracts in schizophrenia. Correlation analyses demonstrated negative relationships between FA in the cerebrum, particularly in the left hemisphere, and SANS global and global rating scores (Anhedonia-Asociality, Attention, and Affective-Flattening), and also associations between FA of left frontal lobe and SANS global score, Anhedonia-Asociality, and Attention. This study demonstrates that patients with chronic schizophrenia evince widespread cerebral FA abnormalities and that these abnormalities, especially in the left hemisphere, are associated with negative symptoms.
Keywords: Schizophrenia, DTI, TBSS, FA, negative symptom, frontal lobe
1. Introduction
Symptoms of schizophrenia are thought to arise from inadequate communication between brain regions, particularly the frontal and temporal lobes. Recent postmortem and genetic studies in schizophrenia provide evidence demonstrating myelin abnormalities that might affect this communication (Hakak et al., 2001; Katsel et al., 2005). These findings support the hypothesis that deficits in structural brain connectivity are evident in schizophrenia and, importantly, that they may constitute a trait maker of pathology.
Previous diffusion tensor imaging (DTI) studies have observed both local and widespread fractional anisotropy (FA) reductions in white matter (WM) regions in schizophrenia compared with healthy control subjects (HC) (for review, see Kubicki et al., 2007), and these abnormalities have been attributed to disruptions in connectivity. Although WM abnormalities have been frequently reported in the schizophrenia literature, their clinical significance remains less studied.
It is thought that negative symptoms become more pronounced in the chronic stage of illness (Tandon et al., 2009). Negative symptoms include flattened affect, social withdrawal, and attentional deficits, and the severity of negative symptoms is associated with impairment in various cognitive functions such as memory, learning, and executive function (Bilder et al., 2000; Sanfilipo et al., 2002). Negative symptoms are also strongly associated with social dysfunction (Tsai et al., 2010) and poor long-term outcome (Javitt, 2001). It is therefore important to elucidate the pathology of negative symptoms in chronic schizophrenia.
Negative symptoms are hypothesized to be the result of structural brain deficits (Crow, 1980). Considering the presumed associations between negative symptoms and various cognitive dysfunctions, we speculate that negative symptoms may be associated with abnormalities in multiple brain regions in chronic schizophrenia. Indeed, previous neuroimaging studies have reported associations between negative symptoms and abnormal gray and white matter volumes in several brain regions, such as the prefrontal cortex (Ho et al., 2003; Wible et al., 2001), thalamus (Yoshihara et al., 2008), precentral cortex, and inferior parietal gyrus (Premkumar et al., 2009), in schizophrenia. These findings led us to investigate WM connectivity abnormalities in various brain regions and their associations with negative symptoms in chronic schizophrenia.
With respect to DTI studies, only a small number of studies have identified associations between negative symptom severity and FA abnormalities in patients with schizophrenia. Such studies, however, focused on either localized WM regions (Wolkin et al., 2003) or specific tracts (Mitelman et al., 2007; Kubicki et al., 2008; Whitford et al., 2010). We believe that it is important to identify which WM regions are most associated with negative symptoms at each anatomical level (i.e., whole brain WM, regional WM, a specific tract), in order to understand further the pathology of negative symptoms in schizophrenia. To the best of our knowledge, there are no studies that have investigated structure-symptom relationships at each of these anatomical levels simultaneously, as is proposed here.
Previous DTI studies of schizophrenia have also reported associations between positive symptoms and localized WM or tracts (Fujiwara et al., 2007; Skelly et al., 2008; Whitford et al., 2010; Whitford et al., 2012; Lee et al., 2013). These findings are, however, inconsistent. For example, some previous studies have shown positive associations between positive symptoms and structural deficits in the regional WM, while others have detected negative relationships between them. Therefore, it is also important to explore associations between positive symptoms and WM at each anatomical level to better understand the neurobiology of positive symptoms in schizophrenia.
Based on the aforementioned postmortem, structural, DTI, and cognitive findings, the goal of the current study was to detect abnormalities in WM integrity and their association with negative symptoms in chronic schizophrenia. The Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006) approach was used in this study. In addition to a standard voxel-based approach, TBSS, when combined with WM atlases, also allows for region of interest (ROI) analysis, where WM of either the entire hemisphere, entire lobe, or an individual fiber bundle can be analyzed (Karlsgodt et al., 2009).
In the current study, we first evaluated FA abnormalities in cerebral and lobar WM in patients with chronic schizophrenia compared with healthy controls (HC). Second, a voxel-wise comparison was conducted to evaluate FA abnormalities along all WM tracts. Finally, relationships were evaluated between WM abnormalities in cerebrum, each lobe, and each tract and clinical symptoms, particularly negative symptoms, in schizophrenia.
2. Methods
2.1. Subjects
Participants comprised 24 patients with chronic schizophrenia and 25 HC. Participants’ recruitment and detailed study criteria were described in our previous report (Choi et al., 2011). Groups were matched in age, handedness, and parental socioeconomic status or premorbid IQ derived from the Wide Range Achievement Test-3 reading scores (Wilkinson, 1993). The mean global scores of the Scales for the Assessment of Negative Symptoms (SANS) and Positive Symptoms (SAPS) (Andreasen, 1983, 1984) were 11.9±7.0/9.3±4.1. Demographic and clinical data are presented in Table1. Of all patients included in this study, two received only typical antipsychotics, 17 patients received only atypical antipsychotics, two received both typical and atypical antipsychotics, and three patients received no antipsychotics at the time of the scan. This study was approved by the local IRB at both the VA and Brigham and Women's Hospital. Written informed consent was obtained from all subjects before study participation.
Table 1.
Demographic and clinical characteristics of the study groups
| SZ group (n=24) |
HC group (n=25) |
||||
|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | dfa | t test | p |
| Age, mean (SD) [range], years | 44.4 (9.7) [22–55] |
40.4 (11.4) [21–55] |
47 | 1.31 | 0.20 |
| Sex, No. M/F | 24 / 0 | 25 / 0 | |||
| Handednessb | 0.71 (0.25) | 0.78 (0.18) | 45 | 1.10 | 0.28 |
| Socioeconomic Statusc | |||||
| Subject’s | 3.5 (1.1) | 2.5 (1.2) | 46 | 2.91 | 0.01* |
| Parental | 2.6 (1.2) | 2.7 (1.3) | 45 | 0.28 | 0.78 |
| WRAT-3: reading score | 97.6 (12.1) | 103.5 (11.6) | 43 | 1.68 | 0.10 |
| Symptom onset, years | 23.9 (5.9) | NA | |||
| Duration of illness, years | 20.5 (10.3) | NA | |||
| Antipsychotic medication dosage (mg/day)d | 346.9 (265.5) | NA | |||
|
Neuroleptics, No. of patients TYP/ATYP/Overlap/Non-medication |
2 / 17 / 2 / 3 | NA | |||
| Number with/without family history of first-degree relatives with psychosise | 3 / 17 | NA | |||
| SANS global score | 11.9 (7.0) | NA | (n = 23) | ||
| SAPS global score | 9.3 (4.1) | NA | (n = 23) |
Abbreviations: SZ, schizophrenia; HC, healthy control subject; WRAT-3, Wide Range Achievement Test-3; SANS, the Scale for the Assessment of Negative Symptoms; SAPS, the Scale for the Assessment of Positive Symptoms; NA, data not applicable; TYP/ATYP, typical/atypical antipsychotics
2.2. MRI protocol
All subjects were scanned on a 3T GE Echospeed system, using an echo planar imaging (EPI) DTI sequence. A double echo sequence with an eight-channel coil was used to reduce eddy-current and EPI (echoplanar imaging) spatial related distortions. Fifty-one noncolinear diffusion directions (B=900) and nine baseline scans (B=0) were acquired with the following scan parameters: repetition time 17000 ms, echo time 78 ms, field of view 24cm, 144×144 encoding steps, and 1.7 mm slice thickness. Eighty-five axial slices spanning the entire brain were collected for each subject.
2.3. Image processing
After reconstruction, diffusion-weighted images were transferred to a LINUX workstation, on which FA and trace maps of the diffusion tensor were calculated.
In this study, TBSS 1.2 (Smith et al., 2006; Smith et al., 2007) was used. FA images were first reoriented using rigid body transformation. Next, the reoriented images were transformed into FMRIB58_FA standard space using a non-linear registration of FMRIB’s Nonlinear Image Registration Tool. A mean FA image was then created by averaging all the registered FA images, and a group skeleton, which represents the center of all tracts common to the entire group, was generated from these images. Finally, all of the registered FA images were projected onto this group skeleton, and a 4D file of all skeletonized images from each subject was created. The FA threshold was set at 0.2 to confine the analysis to the WM.
For whole brain FA skeleton analyses, each individual FA skeleton was created by splitting the 4D file of all FA skeletonized images into individual 3D FA skeleton images. The same skeletons were used for the trace analysis.
2.4. Statistical analysis
2.4.1. Whole brain, cerebral, and lobar FA skeleton analyses
The FA skeleton was manually separated into lobar segments and, then, mean FAs of whole brain, its components (cerebrum, cerebellum, and brain stem), and each lobe (right and left frontal, temporal, parieto-occipital lobes, and subcortical region) were calculated using 3D–Slicer (v2.8, http://www.slicer.org). To determine group differences in mean FA of the global or lobar FA skeleton, we conducted statistical analyses as follows. First, two-sample t-tests were applied with cutoff p values set at 0.05 (for whole brain comparison) and 0.017 (0.05/3 components, for each component’s comparison). Second, a repeated measures analysis of variance (ANOVA) was employed to determine group differences in mean FA of each cerebral hemisphere. Finally, we conducted a two-sample t-test to determine group differences in mean FA of each lobe. In this analysis, the significance level was set at p<0.006 (0.05/8 lobes) and the trend level was set at p<0.05. The same analyses were conducted for group comparisons of trace.
2.4.2. Voxel-wise analysis
A whole brain voxel-wise group comparison was conducted to investigate FA abnormalities in WM tracts between schizophrenia patients and HC. A permutation-based inference tool for nonparametric statistics (Nichols and Holmes, 2002) was used and a two-sample t-test was conducted for all voxel-wise group comparisons using a threshold-free cluster enhancement method (Smith and Nichols, 2009). The number of permutations was set at 5000, and the significance level was set at Family-Wise Error (FWE) corrected p<0.05. The same analysis was conducted for group comparison of trace.
2.4.3. Significant white matter tract identification
Local group differences within individual WM tracts were identified using Mori’s WM atlas (Mori, 2005), which was previously registered to FMRIB58_FA standard space, and mean FAs of each region were calculated using 3D–Slicer.
2.4.4. Correlation analysis
To investigate the relationship between clinical symptoms and mean FAs of cerebrum and lobes in the chronic schizophrenia, we conducted Spearman’s correlation analyses as follows. First, the relationships between the mean FAs of cerebrum and SANS/SAPS global scores were evaluated. Second, correlation analysis was conducted between the FAs of individual hemispheres of the cerebrum and the global scores, as well as global rating scores, to evaluate the effect of hemisphere. Finally, the relationships between the mean FAs of each lobe and global rating scores were evaluated. The significance thresholds were set at p<0.01 for these analyses to correct for multiple comparisons.
In the case of observing significant relationships in the above analyses, exploratory correlational analyses with a cutoff of p<0.05 were conducted between FAs of WM tracts, for which the voxel-wise analysis showed significant FA reductions, and the global rating scores in the schizophrenia group.
Furthermore, correlational analyses were conducted between antipsychotic medication dosages and clinical scores and mean FAs of the cerebral, lobar, and WM tracts with a significance threshold of p<0.01 to evaluate medication effects on symptoms and FAs.
3. Results
3.1. Whole brain, cerebral, and lobar FA skeleton analyses
Results are summarized in Table2. The patient group showed significantly lower mean FAs of the whole brain, especially of the cerebrum but not of the cerebellum and brain stem, compared with the HC. A repeated measures analysis of variance (ANOVA) showed smaller mean FAs for both left and right hemispheres of the cerebrum in the schizophrenia patients compared with the HC.
Table 2.
Mean FA values of the whole brain, cerebral, and lobar FA skeletons
| SZ group (n=24) |
HC group (n=25) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Number of voxels |
Mean FA |
SD | Mean FA |
SD | t value |
df | p (two- tailed) |
% reduction |
| FA skeleton of whole brain (cerebrum, cerebellum, and brain stem) | 129382 | 0.40 | 0.02 | 0.42 | 0.01 | 2.49 | 47 | 0.016*a | 2.7 |
| FA skeleton of cerebrum | 116182 | 0.40 | 0.02 | 0.41 | 0.01 | 2.63 | 47 | 0.014*b | 2.9 |
| FA skeleton of cerebellum | 7315 | 0.37 | 0.02 | 0.38 | 0.01 | 1.41 | 47 | 0.164 | 1.9 |
| FA skeleton of brain stem | 5885 | 0.52 | 0.02 | 0.53 | 0.02 | 0.21 | 47 | 0.832 | 0.4 |
| FA skeleton of cerebrum in left hemisphere | 57512 | 0.40 | 0.02 | 0.41 | 0.01 | 2.66 | 47 | 0.011*c | 2.9 |
| FA skeleton of cerebrum in right hemisphere | 58670 | 0.40 | 0.02 | 0.41 | 0.02 | 2.56 | 47 | 0.011*c | 2.7 |
| FA skeleton of left frontal lobe | 20050 | 0.38 | 0.02 | 0.39 | 0.01 | 3.18 | 47 | 0.003*d | 3.6 |
| FA skeleton of right frontal lobe | 20101 | 0.39 | 0.02 | 0.40 | 0.02 | 2.95 | 47 | 0.005*d | 3.2 |
| FA skeleton of left temporal lobe | 10758 | 0.39 | 0.02 | 0.40 | 0.02 | 2.30 | 47 | 0.026*e | 2.5 |
| FA skeleton of right temporal lobe | 11571 | 0.39 | 0.02 | 0.40 | 0.02 | 2.22 | 47 | 0.031*e | 2.5 |
| FA skeleton of left parietooccipital lobe | 17481 | 0.40 | 0.02 | 0.41 | 0.02 | 2.54 | 47 | 0.015*e | 3.2 |
| FA skeleton of right parietooccipital lobe | 18014 | 0.40 | 0.02 | 0.41 | 0.02 | 2.76 | 47 | 0.008*e | 3.5 |
| FA skeleton of left subcortical region | 9223 | 0.47 | 0.02 | 0.47 | 0.01 | 1.04 | 47 | 0.306 | 1.0 |
| FA skeleton of right subcortical region | 8984 | 0.46 | 0.02 | 0.47 | 0.01 | 1.42 | 47 | 0.162 | 1.4 |
Abbreviations: FA, fractional anisotropy; SZ, schizophrenia; HC, healthy control subject
A two sample t-test with cutoff p value of 0.05 showed significant FA reduction in the schizophrenia compared with the HC.
A two sample t-test with a Bonferoni-corrected cutoff p value of 0.017 (0.05/3 regions) showed significant FA reduction in the schizophrenia compared with the HC.
A repeated measures analysis of variance, post hoc analysis using simple t-test with a Bonferoni-corrected cutoff p value of 0.025 (0.05/2 hemispheres) revealed significant FA reduction of both hemispheres in the schizophrenia compared with HC.
A two sample t-test with a Bonferoni-corrected cutoff p value of 0.006 (0.05/8 regions) showed significant FA reduction in the schizophrenia compared with the HC.
A two sample t-test showed trend level (p <0.05) FA reduction in the schizophrenia compared with the HC.
At the lobar level, the patient group had significantly lower mean FAs of bilateral frontal lobes compared with the HC. The mean FAs of bilateral temporal and parieto-occipital lobes showed trend reductions, and FAs of subcortical regions showed no reduction in the schizophrenia patients compared with the HC. No group differences were found in mean trace of cerebral and lobar skeletons.
3.2. Voxel-wise analysis
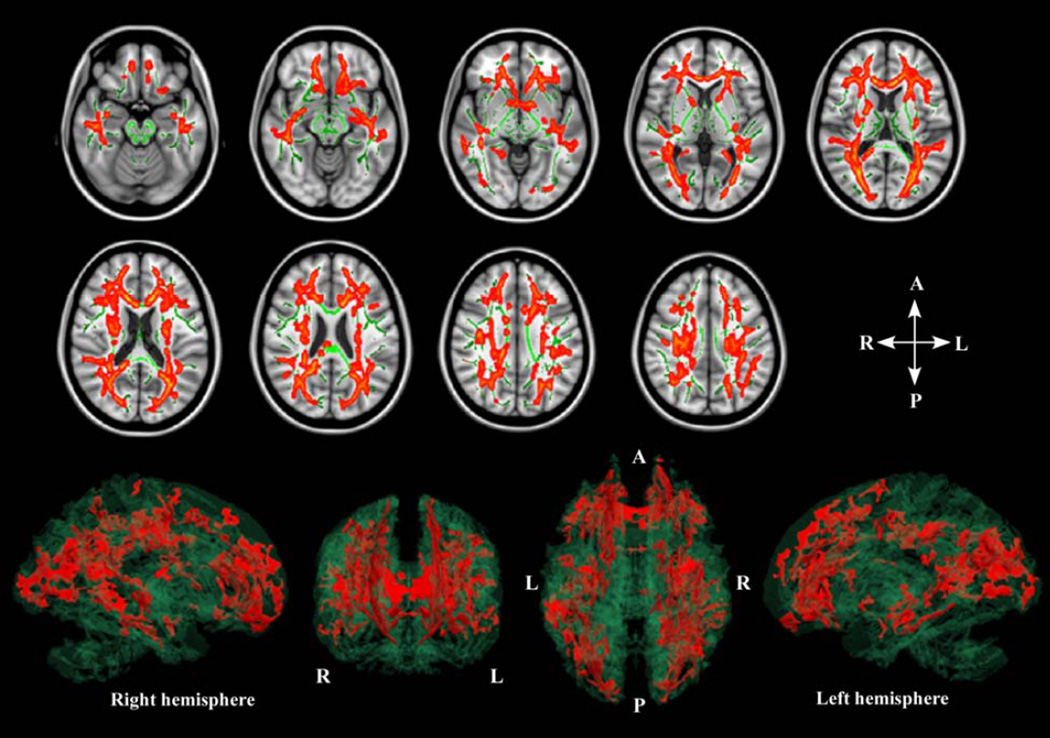
The whole brain voxel-wise analysis showed significant FA reductions in almost all the WM tracts in the cerebrum, but no differences in WM tracts in the cerebellum and brain stem in the schizophrenia patients compared with HC (FWE-corrected p<0.05, Table 3 and Fig. 1). There were neither increases in FA nor trace changes in WM tracts in the schizophrenia patients compared with HC.
Table 3.
Mean FA values of each WM tract where the voxel-wise TBSS analysis showed significant FA reductions in chronic schizophrenia compared with HC
| SZ group (n=24) |
HC group (n=25) |
SZ group (n=24) |
HC group (n=25) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | voxels | Mean FA |
SD | Mean FA |
SD | % reduction |
Region | voxels | Mean FA |
SD | Mean FA |
SD | % reduction |
|
| Association fibers (left hemisphere) | Association fibers (right hemisphere) | |||||||||||||
| Uncinate fasciculus | 70 | 0.37 | 0.03 | 0.40 | 0.05 | 7.2 | Uncinate fasciculus | 30 | 0.38 | 0.04 | 0.41 | 0.06 | 8.6 | |
| Fornix | 93 | 0.42 | 0.03 | 0.46 | 0.05 | 7.7 | Fornix | 113 | 0.45 | 0.02 | 0.48 | 0.03 | 4.5 | |
| Cingulum | 18 | 0.44 | 0.04 | 0.47 | 0.05 | 6.5 | Cingulum | 61 | 0.52 | 0.03 | 0.55 | 0.03 | 5.4 | |
| SLF | 739 | 0.42 | 0.03 | 0.45 | 0.02 | 6.4 | SLF | 852 | 0.40 | 0.03 | 0.42 | 0.02 | 5.9 | |
| ILF | 72 | 0.45 | 0.03 | 0.48 | 0.03 | 6.4 | ILF | 71 | 0.44 | 0.04 | 0.47 | 0.03 | 5.9 | |
| Sagittal stratum | 335 | 0.47 | 0.03 | 0.50 | 0.03 | 5.6 | Sagittal stratum | 290 | 0.46 | 0.03 | 0.48 | 0.03 | 5.0 | |
| SFOF | 36 | 0.48 | 0.03 | 0.50 | 0.03 | 3.6 | SFOF | 39 | 0.37 | 0.04 | 0.40 | 0.05 | 9.2 | |
| IFOF | 42 | 0.44 | 0.04 | 0.47 | 0.04 | 5.8 | IFOF | 90 | 0.43 | 0.03 | 0.46 | 0.04 | 6.0 | |
| Projection fibers (left hemisphere) | Projection fibers (right hemisphere) | |||||||||||||
| Int. capsule | Int. capsule | |||||||||||||
| whole | 593 | 0.52 | 0.03 | 0.54 | 0.02 | 4.4 | whole | 472 | 0.49 | 0.02 | 0.51 | 0.03 | 4.6 | |
| ant. limb | 180 | 0.54 | 0.03 | 0.56 | 0.03 | 4.5 | ant. limb | 205 | 0.46 | 0.03 | 0.49 | 0.03 | 5.3 | |
| post. limb | 89 | 0.53 | 0.02 | 0.55 | 0.03 | 3.2 | post. limb | 41 | 0.56 | 0.02 | 0.58 | 0.03 | 3.3 | |
| retro. part | 324 | 0.50 | 0.04 | 0.52 | 0.03 | 4.6 | retro. part | 226 | 0.50 | 0.03 | 0.52 | 0.03 | 4.2 | |
| Ext. capsule | 174 | 0.43 | 0.03 | 0.46 | 0.02 | 5.9 | Ext. capsule | 215 | 0.39 | 0.02 | 0.41 | 0.02 | 5.0 | |
| ant. corona radiata | 929 | 0.41 | 0.03 | 0.43 | 0.03 | 6.8 | ant. corona radiata | 148 4 |
0.39 | 0.03 | 0.42 | 0.03 | 7.3 | |
| sup. corona radiata | 869 | 0.42 | 0.02 | 0.44 | 0.03 | 5.2 | sup. corona radiata | 658 | 0.40 | 0.03 | 0.42 | 0.03 | 5.5 | |
| post. corona radiata | 487 | 0.43 | 0.03 | 0.45 | 0.02 | 5.7 | post. corona radiata | 190 | 0.39 | 0.03 | 0.41 | 0.02 | 5.6 | |
| post. thalamic radiation | 106 3 |
0.47 | 0.03 | 0.50 | 0.03 | 6.5 | post. thalamic radiation | 874 | 0.46 | 0.03 | 0.49 | 0.03 | 6.3 | |
| Commissural fibers | ||||||||||||||
| Ant. commissure | 106 | 0.39 | 0.06 | 0.44 | 0.06 | 10.5 | ||||||||
| Corpus callosum ant. part (genu and ant. part of body) | 266 4 |
0.49 | 0.02 | 0.51 | 0.02 | 4.6 | ||||||||
| splenium | 850 | 0.51 | 0.02 | 0.53 | 0.02 | 3.9 | ||||||||
Abbreviations: FA, fractional anisotropy; WM, white matter; TBSS, Tract-based spatial statics; SZ, schizophrenia; HC, healthy control subject; SLF, superior longitudinal fasciculus; ILF, inferior longitudinal fasciculus; SFOF, superior fronto-occipital fasciculus; IFOF, inferior fronto-occipital fasciculus; Int., internal; ant., anterior; post., posterior; retro., retrolenticular; Ext., Externa; sup., superior
Fig. 1.
Images in the axial plane were constructed to show significant differences in fractional anisotropy (FA) between groups, where reduced regions in schizophrenia can be observed (orange regions), as well as mean FA skeleton (green lines) and T1-weighted images of the Montreal Neurological Institute template. The result of voxel-wise Tract-Based Spatial Statistics showed significant FA reductions in almost all white matter tracts in the cerebrum in the 24 chronic schizophrenia compared with 25 healthy controls (Family-Wise Error corrected p < 0.05). 3D images; from left to right: lateral view of right hemisphere, frontal coronal view, superior axial view, and lateral view of left hemisphere. The FA reduced regions (orange) and the mean FA skeleton (clear green) were reconstructed in a 3D image using 3D-Slicer.
3.3. Correlational analysis
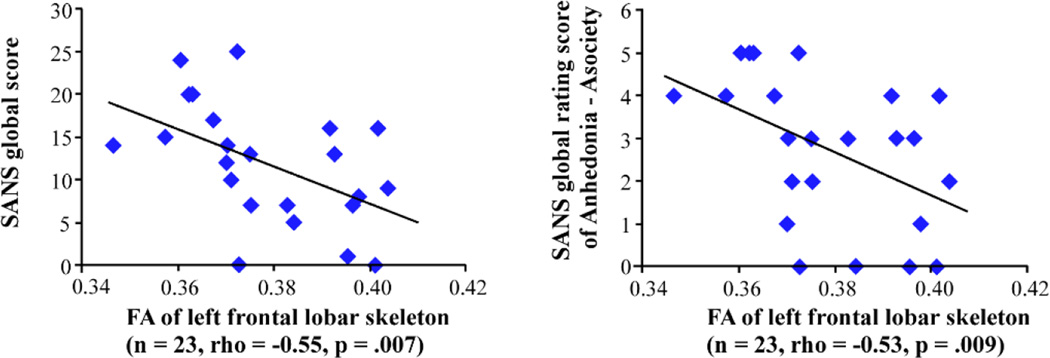
Although the mean FA of the cerebrum showed no statistically significant association with the SAPS global score (rho=0.43, p=0.85), it showed a significant negative association with the SANS global score (results are summarized in Table 4 and Fig. 2). Mean FAs of the left hemisphere cerebrum and frontal lobe evinced significant negative associations with SANS global and some global rating scores, while mean FA of the right hemisphere cerebrum or frontal lobe showed no such associations. FAs of every lobe showed no significant association with SAPS scores.
Table 4.
Results of correlation analyses between cerebral, lobar, and regional FA values and SANS global and global rating scores in patients with chronic schizophrenia
| SANS global score |
Affective Flattening |
Anhedonia- Asociality |
Attention | |||||
|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | |
| cerebrum | −0.56 | 0.005 | ||||||
| L. hemisphere cerebrum | −0.59 | 0.003 | −0.54 | 0.007 | −0.55 | 0.006 | −0.55 | 0.007 |
| L. frontal lobe | −0.55 | 0.007 | −0.53 | 0.009 | −0.54 | 0.008 | ||
| L. Int. capsule, whole | −0.52 | 0.011 | −0.53 | 0.009 | ||||
| L. Int. capsule, ant. limb | −0.48 | 0.021 | −0.44 | 0.034 | ||||
| L. Int. capsule, retro. part | −0.45 | 0.032 | −0.53 | 0.010 | ||||
| L. SFOF | −0.42 | 0.044 | ||||||
| corpus callosum (ant. part) | −0.43 | 0.040 | ||||||
Abbreviations: FA, fractional anisotropy; SANS, the Scale for the Assessment of Negative Symptoms; L., left; Int., internal; ant., anterior; retro., retrolenticular; SFOF, superior fronto-occipital fasciculus;
Fig. 2.
Correlation between fractional anisotropy (FA) values of the frontal lobar skeleton and the Scale for the Assessment of Negative Symptoms (SANS).
According to the results of left hemisphere dominant associations with negative symptoms in the above analyses, exploratory correlation analyses were conducted between FAs of local WM tracts in the left hemisphere and in commissural fibers and Affective Flattening, Anhedonia-Asociality, and Attention subscores. Results showed that the left internal capsule (IC) was negatively correlated with Affective Flattening, Anhedonia-Asociality, and Attention. The left superior fronto-occipital fasciculus (SFOF) was negatively correlated with Affective Flattening, and the anterior part of the corpus callosum was negatively correlated with Anhedonia-Asociality.
Antipsychotic medication dosage was not significantly correlated with mean FAs of the skeletons in cerebrum, frontal lobe or WM tracts, SANS or SAPS scores.
4. Discussion
This study evaluated associations between negative symptoms and WM abnormalities at each anatomical level of the brain (i.e., cerebral WM, local WM tract). Negative symptoms are closely related to social dysfunction in patients with chronic schizophrenia. Therefore, a first step in improving the quality of life for chronic patients is to understand the pathology of negative symptoms. Although negative symptoms are hypothesized to be the result of structural brain deficits, the relationships between WM abnormalities and negative symptoms in schizophrenia are still poorly understood.
To understand the neuropathology underlying negative symptoms, we evaluated structural WM abnormalities in patients with chronic schizophrenia. The whole brain, cerebral, and lobar analyses demonstrated that the patient group showed FA reduction of the whole brain, which was more pronounced in the cerebrum, compared with HC. In the lobar analysis, the patient group showed significantly smaller FAs for bilateral frontal lobes, and trend level FA reductions of the bilateral temporal and parieto-occipital lobes compared with HC. These findings are consistent with previous reports showing FA reductions in bilateral prefrontal, temporal, parietal, and occipital WM regions (Lim et al., 1999; Minami et al., 2003).
The voxel-wise analysis demonstrated significant FA reductions in almost all the WM tracts in the patient group compared with HC. The affected WM tracts included the cingulum, anterior commissure, uncinate fasciculus, corpus callosum, thalamo-frontal pathway, arcuate fasciculus, SFOF and IC. Those results were consistent with our previous DTI studies, which have demonstrated WM abnormalities in chronic schizophrenia using other technologies (such as ROI and tractography) (Kubicki et al., 2002; Kubicki et al., 2003; Kubicki et al., 2005; Kubicki et al., 2008; Rosenberger et al., 2008; Fitzsimmons et al., 2009; Oh et al., 2009; Whitford et al., 2010; Choi et al., 2011).
Results of the FA comparison analyses in this study suggest that widespread neuronal connectivity abnormalities are present in chronic schizophrenia. This notion is also consistent with the postmortem studies reporting myelin abnormalities affecting multiple WM regions (Hakak et al., 2001; Hof et al., 2003; Katsel et al., 2005). When comparing our results in chronic patients with previous results in studies of first episode schizophrenia from our laboratory using the same methodology (Lee et al., 2013), patients with first episode schizophrenia showed widespread FA reductions in the cortical WM regions, but not in the right occipital regions, while chronic patients with schizophrenia showed FA reductions in all the cortical WM regions. In addition, FA reductions in the brain stem were found only in patients with first episode schizophrenia.
Following WM analysis, correlational analyses were conducted to uncover associations of these structural deficits with clinical symptoms in chronic schizophrenia. Previous structural and DTI studies have reported associations between negative symptoms and WM abnormalities in the frontal lobe and in other regions in schizophrenia (Wible et al., 2001; Wolkin et al., 2003; Kubicki et al., 2008; Skelly et al., 2008). Of note, while these previous studies focused only on a single level of WM anatomy, we thought it was important to evaluate structure-symptom relationships at each level of WM anatomy simultaneously to reveal the whole picture of negative symptom pathology. We were also interested in testing whether negative symptoms were associated with WM abnormalities in the left hemisphere or in both hemispheres because some previous studies demonstrated left lateralized associations with negative symptoms (Sigmundsson et al., 2001; Wible et al., 2001).
We first confirmed that the medication dosage had no association with SANS scores and FAs of every region showing significant FA reductions in schizophrenia. The lack of such an association suggested that SANS scores and FAs were likely not affected by medication. Next, we found a significant negative relationship between the SANS global score and FA of the whole cerebrum. In the cerebral hemisphere analysis, FA of the left hemisphere showed significant negative relationships with SANS global score and Affective Flattening, Anhedonia-Asociality, and Attention, while FA of the right hemisphere showed no such relationship. These findings are consistent with previous whole brain voxel-wise analyses that have shown significant volume reductions in widespread WM regions in the left hemisphere (e.g., fronto-temporal regions, parietal region, and subcortical regions) in schizophrenia patients with prominent negative symptoms (Sigmundsson et al., 2001).
In the subsequent analysis, in which cerebral white matter was divided into 10 lobar ROIs (5 in each hemisphere), FA of the left frontal lobe showed strong negative relationships with the SANS global score and Anhedonia-Asociality and Attention subscores, while the right frontal lobe showed no such association. The frontal region controls various functions, such as emotion, motivation, and attention, and dysfunctions of the frontal region have been thought to cause negative symptoms in schizophrenia (Bachmann et al., 2004). Previous MRI and DTI studies have reported associations between negative symptoms and WM volume or FA reductions in frontal regions in schizophrenia (Ho et al., 2003; Wolkin et al., 2003; Mitelman et al., 2007; Mitelman et al., 2009). Although these previous studies used volumes or FAs of left and right combined frontal structures, our current result showed such relationships only in the left frontal lobe. This finding is consistent with our previous study showing negative association between WM volume in the left prefrontal cortex and the SANS global score in chronic schizophrenia (Wible et al., 2001). Our results are also in line with the result of a functional MRI study with medication-naïve first-episode schizophrenia showing a significant relationship between the brain dysfunction in the left dorsolateral prefrontal cortex (DLPFC) and negative symptoms (van Veelen et al., 2010). Moreover, recent studies of the repetitive transcranial magnetic stimulation for schizophrenia have reported that stimulation to the left DLPFC improved negative symptoms in patients with schizophrenia (Prikryl and Kucerova, 2013; Prikryl et al., 2013). These findings suggest that structural and functional abnormalities in the left frontal lobe, along with tracts connecting the left frontal lobe, play an important role in negative symptoms in the patients with schizophrenia.
In the exploratory correlational analysis, negative associations were confirmed between Affective Flattening and Anhedonia-Asociality and the left anterior limb of IC, left SFOF, and corpus callosum (anterior part). Recent neuroimaging studies have demonstrated an association between affective negative symptoms (affective flattening and anhedonia) and dysfunction in the reward system in schizophrenia (Crespo-Facorro et al., 2001). The reward system includes the nucleus accumbens, caudate nucleus, amygdala, and prefrontal cortex, and plays a crucial role in the processing of emotions (Breiter et al., 1997). The anterior limb of the IC has been reported to be anatomically connected with these brain regions (Gutman et al., 2009), and the anterior part of the corpus callosum is thought to be functionally associated with the frontal lobe (Kubicki et al., 2008; Whitford et al., 2010). Moreover, the SFOF connects the frontal and parietal regions through the superior edge of the anterior limb of the IC and caudate nucleus (Mori et al., 2007). Damage to these fibers therefore could result in a dysfunction of the reward system, and this could, in turn, underlie the negative symptoms observed in schizophrenia. Our findings are supported by previous studies that have shown significant WM volume reduction in the anterior limb of the IC in schizophrenic patients with negative symptoms (Sigmundsson et al., 2001), and which demonstrate a negative association between the anterior corpus callosum area and anhedonia (Woodruff et al., 1997). The current findings are also supported by our previous DTI studies, which showed negative associations between the anterior part of corpus callosum and negative symptoms (Kubicki et al., 2008; Whitford et al., 2010).
We also found negative associations between the left retrolenticular part of the IC and Anhedonia-Asociality and Attention subscores. The retrolenticular part of the IC is thought to be a component of the thalamocortical/corticothalamic pathways interconnecting the thalamus and the parietal and occipital regions (Nagae et al., 2007). These gray matter regions have been reported to have associations with anhedonia and attention (Crespo-Facorro et al., 2001; Ojeda et al., 2002).
4.1. Limitations
Our correlational analysis between FAs of WM tracts and global rating scores on the SANS was exploratory in nature; a confirmation of these findings will be necessary in future planned studies. This study should also be followed by hypothesis-driven studies performing DT tractography in native space. Furthermore, even though medication dosage showed no association with SANS scores or FAs of every WM region in our study, it does not mean that medication has no effect on WM. In fact, cumulative effects of medication have been shown to decrease volume of WM in animals (Konopaske et al., 2008), and thus further studies are needed to estimate medication effects in schizophrenia.
In conclusion, by evaluating FA abnormalities or structure-symptom relationships at each level of WM anatomy (cerebrum, lobe, and tract), this study provided evidence that, when compared with HC, schizophrenia patients showed widespread WM abnormalities in the cerebrum. Furthermore, the cerebral WM abnormalities, particularly in the left hemisphere, were associated with negative symptoms observed at the chronic stage of illness.
Acknowledgments
This study was supported, in part, by grants from the National Institute of Health (K05 MH070047 and R01 MH 50740 to MES, R01 MH 40799 to RWM, P50MH 080272-CIDAR award- to RWM, MES), the Department of Veterans Affairs Merit Awards (MES, RWM), the VA Schizophrenia Center Grant (RWM/MES). This work was also, in part, supported by the National Alliance for Medical Image Computing (NA-MIC), the latter a grant supported through the National Institutes of Health Roadmap for Medical Research, Grant U54 EB005149 (MK). TJW is supported by an Overseas-Based Biomedical Training Fellowship from the National Health and Medical Research Council of Australia (#520627), and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD). We thank Jorge L. Alvarado, B.S., Paula E. Pelavin, B.A., and Kathryn J. Hawley, B.A., for their support as research assistants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no competing financial interests.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: The University of Iowa; 1984. [Google Scholar]
- Bachmann S, Bottmer C, Pantel J, Schroder J, Amann M, Essig M, Schad LR. MRI-morphometric changes in first-episode schizophrenic patients at 14 months follow-up. Schizophrenia Research. 2004;67:301–303. doi: 10.1016/S0920-9964(03)00002-1. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. The American Journal of Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Choi H, Kubicki M, Whitford TJ, Alvarado JL, Terry DP, Niznikiewicz M, McCarley RW, Kwon JS, Shenton ME. Diffusion tensor imaging of anterior commissural fibers in patients with schizophrenia. Schizophrenia Research. 2011;130:78–85. doi: 10.1016/j.schres.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Molecular pathology of schizophrenia: more than one disease process? British Medical Journal. 1980;280:66–68. doi: 10.1136/bmj.280.6207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, Shenton ME. Diffusion tractography of the fornix in schizophrenia. Schizophrenia Research. 2009;107:39–46. doi: 10.1016/j.schres.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, Fukuyama H, Sawamoto N, Hayashi T, Murai T. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophrenia Research. 2007;95:215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biological Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Archives of General Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr., Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biological Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Management of negative symptoms of schizophrenia. Current Psychiatry Reports. 2001;3:413–417. doi: 10.1007/s11920-996-0036-9. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophrenia Research. 2005;79:157–173. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biological Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatric Research. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophrenia Research. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. The American Journal of Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophrenia Research. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Archives of General Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Minami T, Nobuhara K, Okugawa G, Takase K, Yoshida T, Sawada S, Ha-Kawa S, Ikeda K, Kinoshita T. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Canfield EL, Newmark RE, Brickman AM, Torosjan Y, Chu KW, Hazlett EA, Haznedar MM, Shihabuddin L, Buchsbaum MS. Longitudinal assessment of gray and white matter in chronic schizophrenia: a combined diffusion-tensor and structural magnetic resonance imaging study. Open Neuroimaging Journal. 2009;3:31–47. doi: 10.2174/1874440000903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, Hazlett EA, Tang CY, Shihabuddin L, Buchsbaum MS. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PCM, Wakana S, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier Books: Oxford; 2005. [Google Scholar]
- Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, Nakabayashi T, Hori H, Harada S, Saitoh O, Matsuda H, Kunugi H. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Research: Neuroimaging. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Nagae LM, Hoon AH, Jr., Stashinko E, Lin D, Zhang W, Levey E, Wakana S, Jiang H, Leite CC, Lucato LT, van Zijl PC, Johnston MV, Mori S. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. American Journal of Neuroradiology. 2007;28:1213–1222. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, Westin CF, Shenton ME. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Human Brain Mapping. 2009;30(11):3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda N, Ortuno F, Arbizu J, Lopez P, Marti-Climent JM, Penuelas I, Cervera-Enguix S. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Human Brain Mapping. 2002;17:116–130. doi: 10.1002/hbm.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Peters ER, Anilkumar AP, Simmons A, Kumari V. Structural magnetic resonance imaging predictors of responsiveness to cognitive behaviour therapy in psychosis. Schizophrenia Research. 2009;115:146–155. doi: 10.1016/j.schres.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl R, Kucerova HP. Can repetitive transcranial magnetic stimulation be considered effective treatment option for negative symptoms of schizophrenia? Journal of ECT. 2013;29:67–74. doi: 10.1097/YCT.0b013e318270295f. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Ustohal L, Prikrylova Kucerova H, Kasparek T, Venclikova S, Vrzalova M, Ceskova E. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophrenia Research. 2013;149:167–173. doi: 10.1016/j.schres.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, Niznikiewicz M, Westin CF, Kikinis R, A JS, McCarley RW, Shenton ME. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophrenia Research. 2008;102:181–188. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Research: Neuroimaging. 2002;116:1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. The American Journal of Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophrenia Research. 2008;98:157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophrenia Research. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lysaker PH, Vohs JL. Negative symptoms and concomitant attention deficits in schizophrenia: associations with prospective assessments of anxiety, social dysfunction, and avoidant coping. Journal of Mental Health (Abingdon, England) 2010;19:184–192. doi: 10.3109/09638230903469277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veelen NM, Vink M, Ramsey NF, Kahn RS. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophrenia Research. 2010;123:22–29. doi: 10.1016/j.schres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophrenia Bulletin. 2012;38:486–494. doi: 10.1093/schbul/sbq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry. 2010;68(1):70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O'Donnell BF, Kikinis R, Jolesz FA, McCarley RW. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Research: Neuroimaging. 2001;108:65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test-3 (WRAT-3) Lutz, FL: 1993. [Google Scholar]
- Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. The American Journal of Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Phillips ML, Rushe T, Wright IC, Murray RM, David AS. Corpus callosum size and inter-hemispheric function in schizophrenia. Schizophrenia Research. 1997;23:189–196. doi: 10.1016/s0920-9964(96)00103-x. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Annals of General Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]