Figure 7.
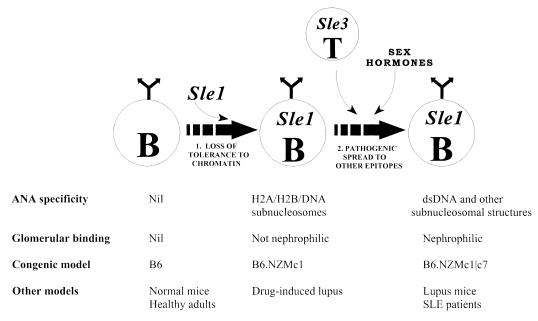
Recipe for nephrophilic autoantibodies in lupus. The data presented in this communication lends support to a two-step recipe for generating nephrophilic autoantibodies and a multi-step model of lupus pathogenesis, as detailed in Discussion. The first step, in effect, breaches tolerance to chromatin. Sle1 is a prototype gene that has the potential to effect this. Importantly, Sle1 triggers the formation of non-nephrophilic anti-H2A/H2B/DNA ANAs in a B cell–intrinsic fashion (53). Although the phenotype in the B6.NZMc1 mice closely resembles the clinical phenotype observed in drug-induced lupus, it is by no means clear if they share underlying pathogenic mechanisms (54, 55). A second set of genes (as exemplified by Sle3, possibly expressed by T cells) and female sex hormones might then act to facilitate the pathogenic maturation of the initial ANA response, leading to nephrophilicity. It is tempting to speculate that the aberrant B cells and T cells triggered by these genetically distinct pathways might be engaged in enhanced bidirectional T-B collaboration, leading to the exaggerated lymphocyte hyperactivity observed in this strain (Figures 2–4 and Table 2). Although the variegated, nephrophilic autoantibody response generated by the combined impact of these 2 sets of genes may be sufficient to inflict progressive renal disease, susceptibility to end-organ damage may be under the control of additional genetic events.