Figure 4.
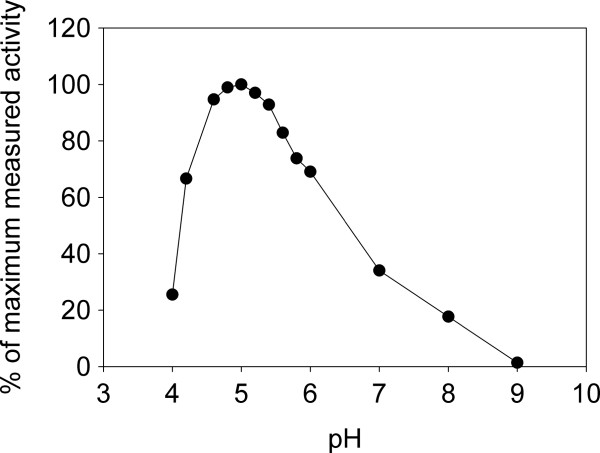
Activity of recombinant T. vaginalis β-fructofuranosidase as a function of pH. Purified β-fructofuranosidase (~27 nM, final concentration) was incubated at the indicated pH in the presence of sucrose and the initial rate of sucrose hydrolysis was determined. The maximum rate of hydrolysis was achieved at pH 5.0. To achieve a pH between 4.0 and 5.8, 20 mM sodium acetate buffer was used; for a pH between 6.0 and 8.0, 20 mM sodium phosphate buffer was employed; and pH 9.0 was reached using 20 mM Tris buffer. All buffers were supplemented with 50 mM NaCl. The results shown are the mean of two independent determinations, each performed in duplicate.
