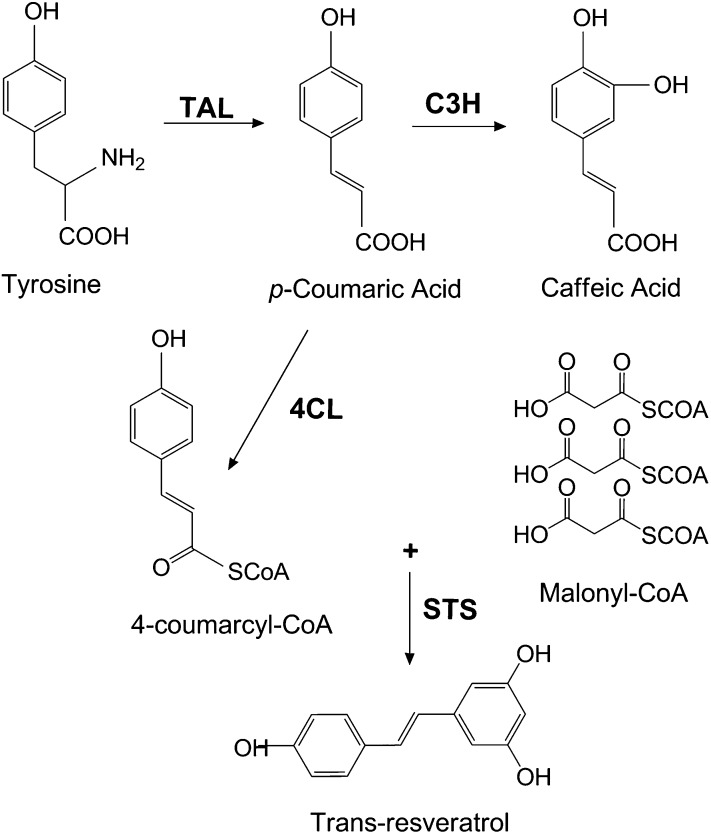
Fig. 1.
Partial phenylpropanoid biosynthetic pathway. Tyrosine is the starting compound for phenylpropanoid biosynthesis. p-Coumaric acid, an intermediate of other phenylpropanoids, is formed by TAL. p-Coumaric acid is further converted into 4-coumarcyl-CoA in the presence of 4CL. Three malonyl-CoA molecules are added to 4-coumaroyl-CoA by an STS enzyme to form trans-resveratrol. C4H, cinnamate-4-hydroxylase; PAL, phenylalanine ammonia lyase.