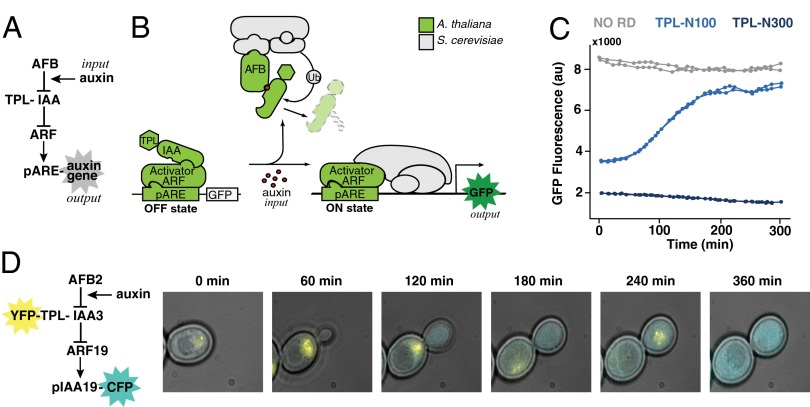
Fig. 1.
Auxin-induced transcription in yeast. (A) Network diagram of the forward auxin response pathway in yeast. An auxin input increases association of a member of the TIR1/AFB family of F-box proteins (AFB) and an IAA protein fused to the first 100 aa of the TPL corepressor (TPL-IAA). Auxin-induced association of an AFB and a TPL-IAA leads to degradation of the TPL-IAA, thereby freeing a transcriptional activator in the ARF family to induce expression of an output gene driven by a promoter containing an auxin response element (pARE). (B) The five A. thaliana components needed to recapitulate auxin response in S. cerevisiae are shown in light green. They were an AFB F-box receptor, an IAA, a TPL corepressor, an ARF transcription factor, and an auxin-responsive promoter. The remaining cellular machinery (gray) was supplied by yeast. Fluorescence from a GFP reporter was used as a quantitative output. (C) Synthetic auxin-reversible repression required fusion of a specific TPL truncation to the IAA protein. Flow cytometry was used to monitor the induction of a GFP reporter following auxin treatment in circuits containing either IAA14 with no repression domain (NO RD), shown in gray, or two different C-terminal TPL truncations fused to IAA14. Auxin was added at time 0. TPL-N300 (dark blue) includes the first 300 aa of TPL and excludes the multiple C-terminal WD repeats. Fusion of TPL-N300 to IAA proteins results in reporter repression that is largely auxin-insensitive. TPL-N100 (light blue) includes only the first 100 aa of TPL. When fused to an IAA, TPL-N100 provides auxin-reversible repression. Two replicate induction curves are shown for each circuit. (D) Auxin-induced IAA degradation and subsequent transcriptional activation could be simultaneously monitored in dual-labeled yeast strains. YFP-TPL-IAA3 and a Cerulean reporter driven by the auxin-responsive IAA19 promoter (pIAA19-CFP) were monitored following auxin treatment using time-lapse microscopy and a microfluidic chamber.