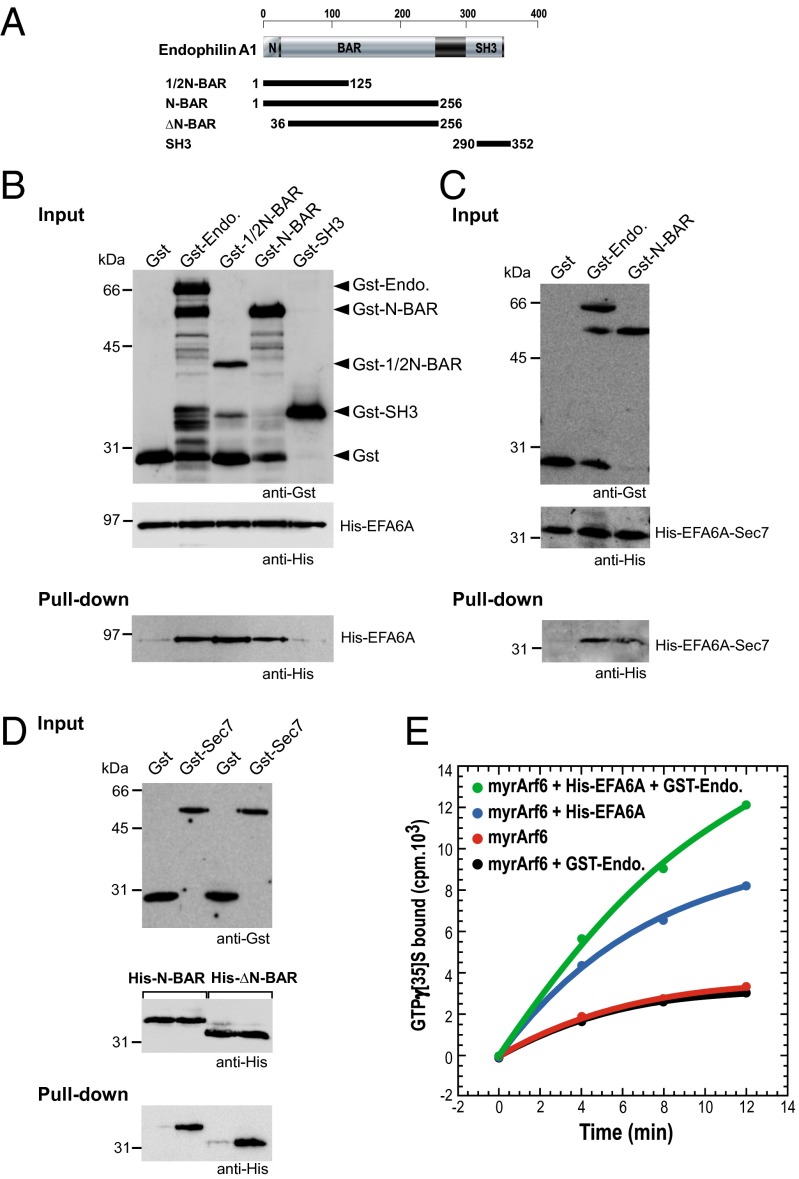
Fig. 1.
Endophilin directly interacts with EFA6 and stimulates its GEF activity on Arf6. (A) Schematic representation of endophilin A1 and the different GST constructs used in this study. (B and C) GST pull-down of purified His-tagged EFA6A (B) or EFA6A-Sec7 domain (C) by different constructs of endophilin A1 fused to GST. (D) GST pull-down of purified His-tagged N-BAR or ΔN-BAR domain by EFA6-Sec7 domain fused to GST. (E) Kinetics of [35S]GTPγS binding to purified and myristoylated Arf6 (2 μM) were measured (Methods) in the presence of phospholipid vesicles and in the presence or the absence of purified His-tagged EFA6A (∼200 nM) and endophilin A1 fused to GST (2 μM). The results are representative of at least three independent experiments.