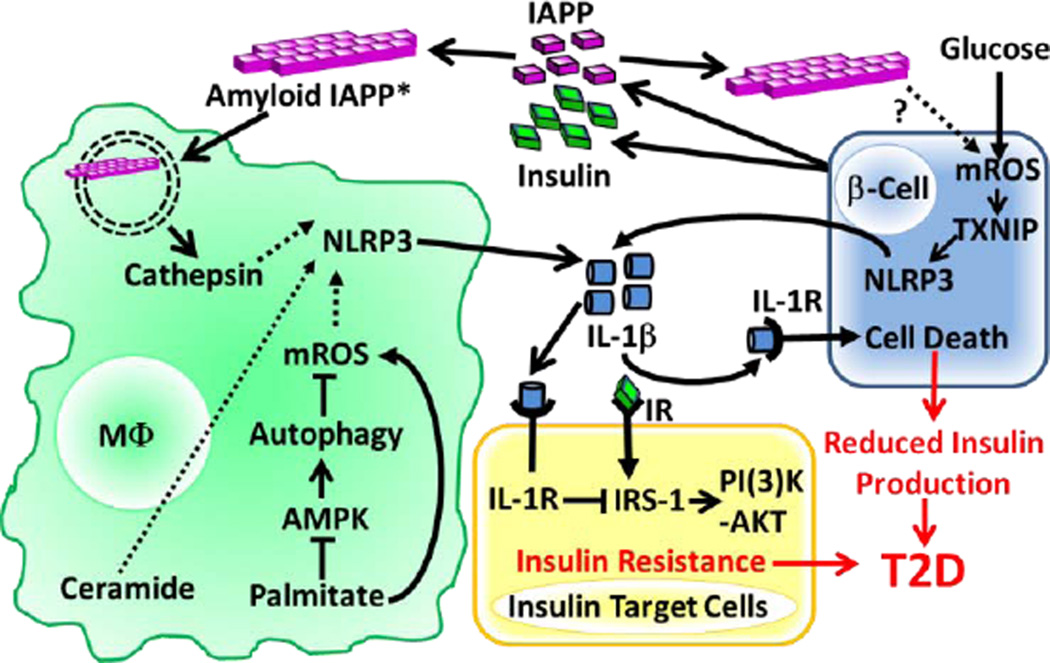
Figure 2. NLRP3 Inflammasome Activation in Response to T2D-associated Metabolic DAMPs.
IAPP is co-secreted with insulin from pancreatic β-cells and the amyloid form of IAPP is internalized by macrophages (soluble IAPP can also become the amyloid form via CD36 mediated internalization*) and destabilizes the phagolysosome resulting in cathepsin release. Palimitate was shown to increase mROS by reducing autophagy through blockade of AMPK signaling. The precise mechanisms by which mROS, cathepsins and ceramide activate the NLRP3 inflammasome remain unknown (dashed lines). Glucose and possibly IAPP can also stimulate NLRP3 activation in β cells by a mechanism that involves mROS and TXNIP activation. Release of IL-1β downstream of NLRP3 activation induces β cell death and blocks insulin receptor (IR) signaling in insulin target cells leading to T2D. (High serum glucose levels are hypothesized to contribute to T2D by maximizing Il1b mRNA expression through the ‘Warburg Effect’ downstream of glycolysis/HIF1α signaling (not depicted).