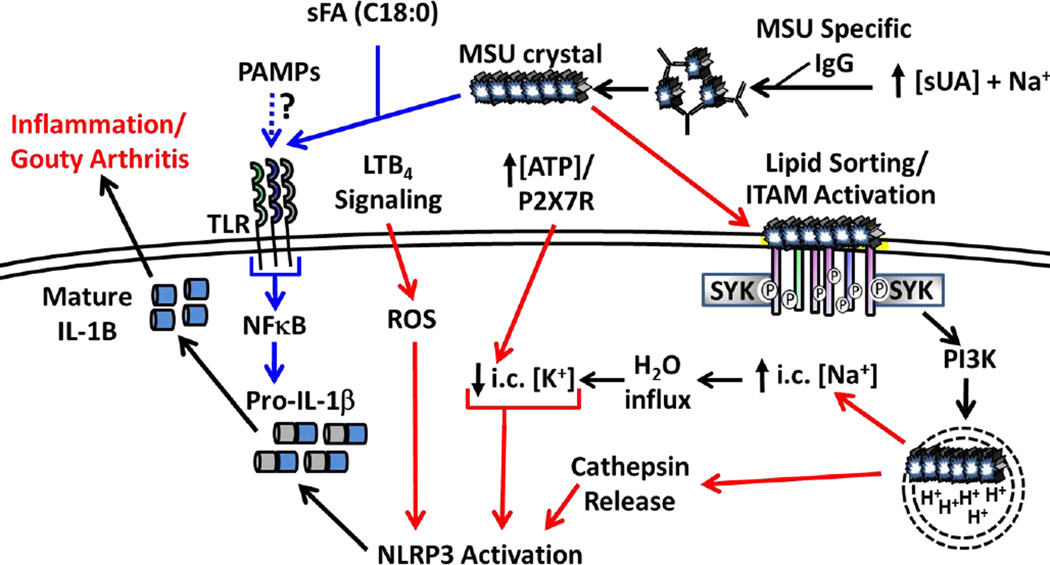
Figure 3. NLRP3 Activation Downstream of MSU Crystal Formation.
High serum UA levels combined with Na+ ions allows for MSU crystallization, which can be facilitated by MSU specific IgG. MSU crystals prime the inflammasome (signal 1, blue arrows) though PRRs (TLR2, TLR4, CD14) and is enhanced in the presence of select sFA (e.g. C18:0). Induction of pro-IL1β may also occur in response to transient PAMP exposure during infection. MSU binding directly to the plasma membrane activates ITAM-containing receptors in a process called “lipid sorting” followed by binding of adaptor molecules (e.g. SYK) that trigger enhanced MSU uptake. Break down of MSU in acidified endosomes is hypothesized to increase intracellular Na+ levels, draw H2O into the cell and effectively lower the apparent intracellular K+ concentration resulting in NLRP3 inflammasome activation (signal 2, red arrows). High ATP concentrations correlating with high sUA levels may enhance K+ efflux via the P2RX7 receptor. MSU crystals can also rupture the phagolysosome resulting in cathepsin release combined with ROS downstream of LTB4 signaling leading to inflammasome activation. The combined effects result in processing and secretion of mature IL-1β, which promotes local inflammation associated with gouty arthritis.