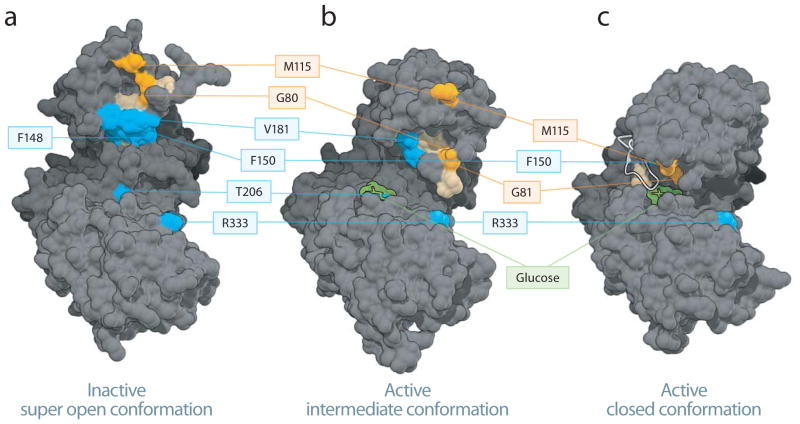
Figure 3.
Mapping the phospho-BAD BH3 helix at the GK interaction site. (a–c) Phospho-BAD BH3 peptides bind to residues in the small domain of the inactive super open and the active intermediate, with the N-terminus localized around residue M115 and the C-terminus oriented toward residues V181, T206 and R333. The most frequently crosslinked residues for each BAD SAHB (≥25% threshold) are mapped onto the inactive super open (a), active intermediate (b), and active closed (c) conformers of GK. Residues selectively crosslinked by BAD SAHBA (Y110Bpa S118pS) and (S118pS D123R F125Bpa) are colored orange and blue, respectively, whereas residues crosslinked by both photoreactive BAD SAHBs are colored tan. For clarity, all of the associated residues are colored, but not all are labeled. Crosslinked residues common to both peptides are among those listed in Supplementary Table 3. Glucose is depicted in green, the Active Site Loop in white.