Abstract
Inflammatory pathway plays an important role in tumor cell progression of colorectal cancers. Although colon cancer is considered as one of the leading causes of death worldwide, very few drugs are available for its effective treatment. Many studies have examined the effects of specific COX-2 and 5-LOX inhibitors on human colorectal cancer, but the role of isothiocyanates (ITSCs) as COX–LOX dual inhibitors engaged in hyaluronan–CD44 interaction has not been studied. In the present work, we report series of ITSC analogs incorporating bioisosteric thiosemicarbazone moiety. These inhibitors are effective against panel of human colon cancer cell lines including COX-2 positive HCA-7, HT-29 cells lines, and hyaluronan synthase-2 (Has2) enzyme over-expressing transformed intestinal epithelial Apc10.1Has2 cells. Specifically, our findings indicate that HA-CD44v6-mediated COX-2/5-LOX signaling mediate survivin production, which in turn, supports anti-apoptosis and chemo-resistance leading to colon cancer cell survival. The over-expression of CD44v6shRNA as well as ITSC treatment significantly decreases the survival of colon cancer cells. The present results thus offer an opportunity to evolve potent inhibitors of HA synthesis and CD44v6 pathway and thus underscoring the importance of the ITSC analogs as chemopreventive agents for targeting HA/CD44v6 pathway.
Keywords: Isothiocyanate analogs, CD44/hyaluronan, Colon cancer, Molecular modeling, COX–LOX dual inhibition, Anti-cancer
Introduction
Although colon rectal cancer (CRC) is the third leading cause of cancer death in men and women in the United States (Siegel et al., 2011), the cellular and molecular events associated with its pathogenesis are not known to a certainty. Epidemiological studies indicate that consumption of green leafy vegetables, fruits, broccoli, and cruciferous vegetable is correlated with a lowered risk of colon cancer (Lin et al., 1998; Steinmetz and Potter, 1991). Sulforaphane (SFN) is the predominant isothiocyanate (ITSC) found in these cruciferous vegetables as its glucosinolate precursor, which has been shown to inhibit colonic aberrant crypt foci (Chung et al., 2000; Kassie et al., 2003). The compound has also been found to be effective inhibitor of carcinogen-induced mammary gland tumorigenesis (Zhang et al., 1994), lung cancer (Hecht et al., 2002), and stomach tumors in rodent model (Fahey et al., 2002). Although mechanism of tumor inhibition by SFN is not clear, it has been reported to affect several cellular processes, including apoptosis (Chen et al., 2012), cell cycle arrest (Davis et al., 2009), disruption of oncogenic signaling (Li et al., 2011), and epigenetic regulation of tumor suppressor genes (Bhamre et al., 2009; Clarke et al., 2008; Dashwood and Ho, 2008; Telang et al., 2009).
Despite extensive research, the pathological and molecular markers for prognosis of colon cancer are still lacking. There is now adequate evidence to indicate that cyclooxygenase-2(COX-2) metabolite prostaglandin-E2 (PGE2) and 5-lipoxygenases (5-LOX) metabolite leukotrienes (LTs) play an important role in colon carcinogenesis (Reddy and Rao, 2002; Reddy et al., 2000; Kawamori et al., 1998; Swamy et al., 2003; Bertagnolli et al., 2006; Soumaoro et al., 2006; Ye et al., 2005; Ihara et al., 2007). The COX-2 transcription is regulated through transcription factors, especially nuclear factor-κB (NF-κB) (Tsatsanis et al., 2006). NF-κB is a ubiquitous and pleiotropic transcription factor closely linked with carcinogenesis by a variety of pathways including cell cycle progression, apoptosis, invasion, metastasis, and inflammation (Bubici et al., 2006). SFN has been shown to possess anti-inflammatory activity, resulting in down-regulation of COX-2 expression (Shan et al., 2009), probably through inhibition of NFκB-DNA binding and transactivation of NFκB-dependent genes (Heiss et al., 2001). Attempts are underway in identifying molecules that are specifically expressed by epithelial tumor cells which correlate with tumor growth and drug resistance. Among such molecules hyaluronic acid (HA), is a major extracellular matrix (ECM) component (Misra et al., 2009) whose interaction with the receptor variant isoform CD44v6 (Aruffo et al., 1990) has been suggested to play a crucial role in regulating COX-2/PGE2 mediated cell survival, motility, and drug resistance (Lesley et al., 2000a, b; Lesley and Hyman, 1992; Naor et al., 2002; Yamada et al., 2004). We had earlier demonstrated that the reversal of HA/CD44v6 signaling can modulate the cancer phenotype and adenoma growth in Apc Min/+ mice by inhibiting CD44v6/ErbB2/COX-2-PGE2 pathway (Ghatak et al., 2008, 2010), suggesting the potential of HA/CD44v6 as target for anti-cancer/chemopreventive drugs. In a recent study, we also demonstrated that CD44v6 is a direct target of COX-LOX dual inhibitors (Misra et al., 2013). The regulation of COX-2 expression and NFκB by SFN has been primarily investigated in inflammation wherein it has been shown that CD44v6 has a binding site for NFκB (Damm et al., 2010). However, the inter-relationships among COX-2, NFκB, and CD44v6 in CRC cells and the mechanism by which SFN modulates these enzymes or protein complexes has not been adequately explored. Recent studies seem to suggest that SFN offers protection against tumor development during the “post initiation” phase. While studying the inhibitory mechanisms by SFN, it has become apparent that this natural compound is an inhibitor of HA/CD44v6-induced cell growth and proliferation as well as COX-LOX enzyme activity. Although bioavailability of SFN in the colon is unknown, a recent pilot study in human mammary tissue has indicated that an oral dose of broccoli sprout preparation containing 200 mmol SFN 1 h prior to tissue removal showed mean accumulation of 1.45 ± 1.12 pmol/mg tissue in the right breast and 2.00 ± 1.95 pmol/mg in the left breast (Cornblatt et al., 2007).
A number of cell-culture studies have been reported in the literature in order to elucidate the mechanism responsible for toxicity, enzyme modulation capability, and metabolic pathways of available isothiocyanates such as SFN, benzylisothiocyanate (BITSC), allylisothiocyanate (AITC), and phenylethyl isothiocyanate (PEITC). These studies revealed that the effects of these ITSCs in cell cultures can vary depending upon the cell line used and experimental conditions employed. The evaluation of these ITSCs in the chemoprevention of breast and other cancers has revealed that these compounds once diffused passively into the cell undergo metabolic conversion pre-dominantly to the glutathione (GSH) conjugate. Of the standard ITSCs available in the market, SFN was found to be the weakest substrate for glutathione S-transferase as reflected from the half-life of their glutathione conjugates at pH 7.4 which are being in the order: PEITC-SG (58 min) ≈ SFN-SG (58 min) < BITC-SG (67 min) (Lamy et al., 2011). Consistent with these findings, the growth inhibition of different cancer cells was found to be in the order of BITC ≈ PEITC > AITC > SFN which also correlates well with their LogP values being in the order: ITSCs (SFN (0.72) < AITSC (2.3) < BITSC (2.97) < PEITSC (3.08) (Lamy et al., 2011). Although this information is important, the absence of commercially available ITSC analogs with various chain lengths and substituents influencing bioactivity of ITSCs impedes reliable conclusions on real availability of isothiocyanate for the cells under study both in vitro and in vivo. With this in mind, we synthesized ITSC analogs with various substituents and structural motifs which can influence the bioactivity of these analogs. Such modifications are also anticipated to increase lipophilicity and LogP values of these compounds and influence their reactivitywith thiol moieties and consequently increase chemical stability (Van Eylen et al., 2007).
In spite of such studies analogs of ITSC which possess better thermal stability than SFN have remained inadequately explored for the inhibition of colon cancer. In the present study we have, therefore, synthesized novel ITSC analogs by incorporating the analogous thiosemicarbazone motif into some of the naturally occurring bioactive phytochemical motifs such as chromone, coumarin, indole, quinolone, and pyridine, respectively. The efficacies of these conjugates in inhibiting CRC cell proliferation and growth as well as sensitizing such cells for apoptosis through CD44v6 and HA pathways have also been examined. Our results suggest that these compounds may represent a novel class of chemopreventive agents for colon cancer by inhibiting the HA/CD44v6/COX-2-5-LOX pathway.
Results and Discussion
Chemistry
Characterization details for synthesized compounds
FPYITSC [(E)-1-((pyridin-2-yl)methylene)thiosemicarbazide]
IR(ν, cm−1): 1725 (C=O), 1617 (C=N imine), 3395 and 3353 (−NH2 free), 3257 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.08 (2H, s, NH2), 7.7 (1H, s, −NH), 8.13 (1H, s, −CH), 8.35 (1H, ArH), 8.37 (1H, ArH), 8.58(1H, ArH), 8.76(1H, ArH), ESI–MS: m/z found 179, Calc 180 (M−) in accordance with C7H8N4S; Anal. Calc. (Found %): C7H8N4S; C, 46.68 (46.65), H, 4.44 (4.47), N, 31.07 (31.09), S, 17.72 (17.79).
APYITSC [(E)-1-(1-(pyridin-2-yl)ethylidene)thiosemicarbazide]
IR(ν, cm−1): 1729 (C=O), 1612 (C=N imine), 3348 and 3306 (−NH2 free), 3231 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.07 (2H, s, NH2), 2.4 (3H, s, −CH3), 7.8 (1H, s, −NH), 8.35 (1H, ArH), 8.41 (1H, ArH), 8.77 (1H, ArH), 10.76 (1H, ArH), ESI–MS: m/z found 193, Calc 194 (M−) in accordance with C8H10N4S; Anal. Calc. (Found %): C8H10N4S; C, 49.44 (49.46), H, 5.22 (5.19), N, 28.85 (28.84), S, 16.45 (16.51).
QNLITSC [(E)-1-((quinolin-2-yl)methylene)thiosemicarbazide]
IR(ν, cm−1): 1719 (C=O), 1619 (C=N imine), 3471 and 3401 (−NH2 free), 3249 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.08 (2H, s, NH2), 7.75 (1H, s, −NH), 7.9 (1H, s, −CH), 8.11 (1H, ArH), 8.20 (1H, ArH), 8.31 (1H, ArH), 8.57 (1H, ArH), 8.68 (1H, ArH), 8.77(1H, ArH) ESIMS: m/z found 229, Calc 230 (M−) in accordance with C11H10N4S; Anal. Calc. (Found %): C11H10N4S; C, 57. 31 (57.37), H, 4.36 (4.38), N, 24.36 (24.33), S, 13.98 (13.92).
CHRITSC [(1E)-1-((4-oxo-4H-chromen-3-yl)methylene) thiosemicarbazide]
IR(ν, cm−1): 1706 (C=O), 1641 (C=N imine), 3477 and 3431 (−NH2 free), 3243 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.06 (2H, s, NH2), 7.53 (1H, s, −NH), 7.68 (1H, s, −CH), 7.79 (1H, ArH), 8.08 (1H, ArH), 8.17 (1H, ArH), 9.15 (1H, ArH), 11.55 (1H, ArH), ESI–MS: m/z found 246, Calc 247 (M−) in accordance with C11H9N3O2S; Anal. Calc. (Found %): C11H9N3O2S; C, 53.41 (53.43), H, 3.59 (3.67), N, 16.94 (16.99), O, 12.92 (12.94) S, 12.93 (12.97).
COUITSC [(1E)-1-(1-(2-oxo-2H-chromen-3-yl)ethylidene) thiosemicarbazide]
IR(ν, cm−1): 1718 (C=O), 1603 (C=N imine), 3471 and 3381 (−NH2 free), 3236 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.06 (2H, s, NH2), 2.25 (3H, s, CH3) 7.40 (1H, s, −NH), 7.60 (1H, s, −CH), 7.75 (1H, ArH), 8.0 (1H, ArH), 8.46 (1H, ArH), 10.45 (1H, ArH), ESI–MS: m/z found 260, Calc 261 (M−) in accordance with C12H11N3O2S; Anal. Calc. (Found %): C12H11N3O2S; C, 55.19 (55.16), H, 4.20 (4.24), N, 16.14 (16.08), O, 12.29 (12.25) S, 12.23 (12.27).
INDITSC [(E)-1-(1-(1H-indol-3-yl)ethylidene)thiosemicarbazide]
IR(ν, cm−1): 1725 (C=O), 1656 (C=N imine), 3577 and 3554 (−NH2 free), 3254 (−NH−); 1H-NMR (CDCl3, δ, ppm): 2.08 (2H, s, NH2), 2.34 (3H, s, CH3) 7. 10 (1H, s, −NH), 7.39 (1H, s, −CH), 7.21 (1H, ArH), 7.91 (1H, ArH), 8.17 (1H, ArH), 10.08 (1H, ArH), 11.53 (1H, −NH heterocyclic) ESI–MS: m/z found 231, Calc 232 (M−) in accordance with C11H12N4S; Anal. Calc. (Found %): C11H12N4S; C, 56.85 (56.87), H, 5.26 (5.21), N, 24.09 (24.12), S, 13.77 (13.80).
Molecular Docking Studies
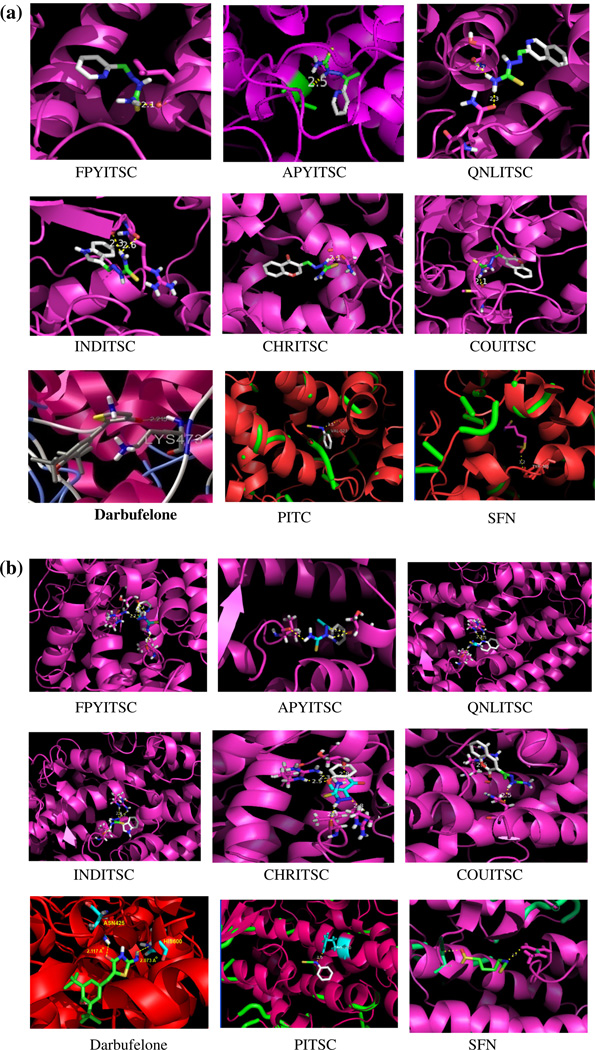
In order to evaluate the efficacy of the synthesized ITSC analogs to inhibit COX-2 activity, they were docked into the cavity of crystallized COX-2 protein from RSPDB (Royal Society Protein Data Bank) http://www.rscb.org/ PDB ID (1PXX). All calculations were performed using AutoDock-Vina software (Trott and Olson, 2010). Grid maps of 50 × 50 × 50 points centered on the active site of the ligand were calculated for each atom types found on the adducts. The AutoDock-Vina program which is an automated docking program was used to dock all ligand molecules in the active site of COX-2 enzyme. For each compound, the most stable docking model was selected based upon confirmation of best score predicted by AutoDock scoring function. The compounds were energy minimized with MMFF94 force field. From the histogram relevant parameters such as binding energy, total number of hydrogen bonds formed, and hydrogen bonding pattern were determined using defined sets of descriptors and adherence to Lipinski’s criterion (Fig. 1a, b). It was observed that the ligand QNLITSC and COUITSC showed best fit in the COX-2 protein cavity with binding energies of −7.80 and −7.4 kcal/mole (Table 1), respectively. The standard COX-LOX dual inhibitor Darbufelone shows (Table 1) slightly less binding energy (−7.08 kcal/mole), whereas far less binding energies were observed for other isothiocyanates like PEITSC (−5.4 kcal/mole) and SFN (−4.5 kcal/mole), respectively. Among the present analogs, QNLITSC having the highest binding energy exhibited two H-bonding interactions involving GIN192 and SEK353 residues, while the next best analog, viz. COUITSC, showed only one H-bonding interaction with MET522 (Table 1).
Fig. 1.
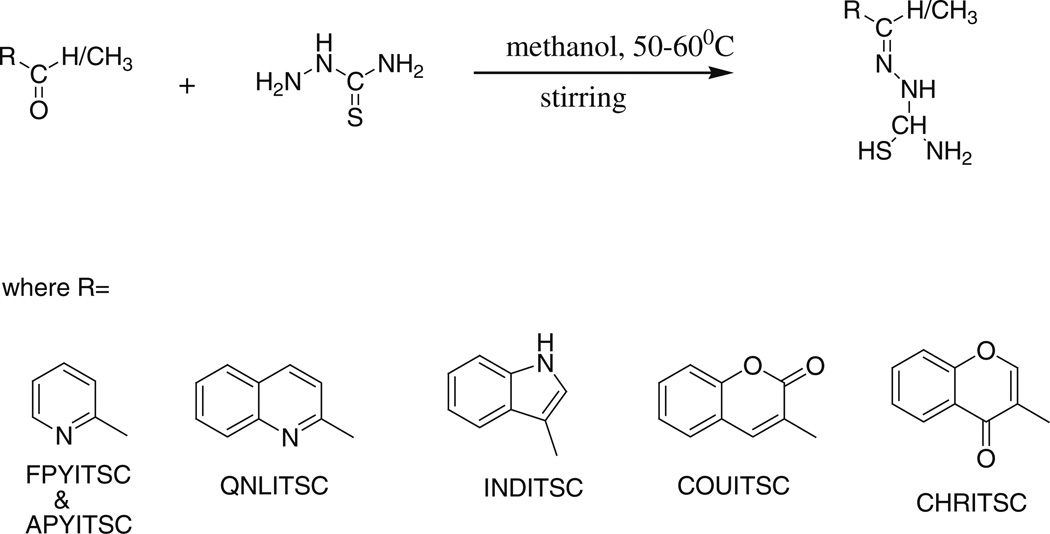
Synthetic scheme for ITSC analogs
Table 1.
Docking results and consensus scores of ITSC analogs in COX-2 protein cavity
| Molecule | BE | Number of H bond |
H bond formed with |
H-bond length |
|---|---|---|---|---|
| FPYITSC | −5.3 | 1 | Leu352 | 2.4 |
| APYITSC | −5.6 | 1 | Val523 | 2.5 |
| QNLITSC | −7.8 | 2 | GLN192 | 2.2 |
| SER353 | 2.3 | |||
| INDITSC | −6.8 | 2 | ARG44 | 2.3 |
| GLY45 | 2.6 | |||
| CHRITSC | −7.3 | 1 | ASN382 | 2.1 |
| COUITSC | −7.4 | 1 | MET522 | 2.1 |
| Darbufelone | −7.08 | 1 | LYS437 | 2.2 |
| PITC | −5.4 | 1 | VAL523 | 3.5 |
| SFN | −4.5 | 1 | TYR385 | 3.2 |
BE binding energy (kcal/mol), H bond hydrogen bond
The X-ray crystal structure of human 5-lipoxygenase protein (5-LOX) has become available only in 2011 due to Gilbert and co-workers (Gilbert et al., 2011).It was obtained from Protein Data Bank (PDB) having PDB ID as 3O8Y and used for the present docking studies of present analogs. The active site residues were obtained from the study of PDB sum (Laskowski, 2009). The ligands were energy minimized and partial charges were added using PRODRG algorithms (Schuttelkopf and van Aalten, 2004). The Auto-Dock Tools graphical user interface (Sanner, 1999) was used to add polar hydrogens and partial charges to 5-LOX Atomic solvation parameters using Kollman United charges and fragment volumes which were assigned using the ADDSOL subroutine. The grid map was calculated using the auxiliary program Autogrid-3. Grid maps of 60 × 60 × 60 points centered on the active site of the ligand with 0.375 Å spacing were calculated for each atom type found on the adducts. Lamarckian Genetic Algorithm (LGA) was selected for ligand conformational search. The Genetic Algorithm (GA) population size was set to 150, the maximum number of GA energy evaluations as 2,500,000, GA mutation rate as 0.02, GA crossover rate as 0.8, and GA docking runs was set as 100. The resulting docking conformations were clustered into families of similar conformation, with root mean square deviation (RMSD) clustering tolerance as 1.0 Å . The lowest docking energy conformations were included in the largest cluster as a rule. Flexible torsion in the ligands was assigned with AUTOTORS, an auxiliary module for Auto-Dock Tools. Each ligand was docked individually within 5-LOX protein cavity to obtain the best binding conformation. The docking results of all compounds in COX-2 and 5-LOX cavities are summarized in Tables 1 and 2. Among the present series CHRITSC, COUITSC, and QNLITSC compounds showed higher binding energies than standard COX-LOX dual inhibitor, Darbufelone. Overall, these findings indicate that the new analogs have higher stability in the COX and LOX protein cavities than the natural isothiocyanate SFN and it is commonly employed analog PEITSC.
Table 2.
Docking results and consensus scores of ITSC analogs in 5-LOX protein cavity
| Molecule | BE | Number of H bond |
H bond formed with |
H-bond length |
|---|---|---|---|---|
| FPYITSC | −5.5 | 3 | ARG370 | 2.2 |
| SER447 | 2.4 | |||
| VA243 | 2.5 | |||
| APYITSC | −6.1 | 2 | VAL243 | 2.7 |
| SER447 | 2.4 | |||
| QNLITSC | −7.3 | 3 | VAL243 | 2.3 |
| ARG370 | 2.7 | |||
| ARG370 | 2.5 | |||
| INDITSC | −6.9 | 3 | ARG370 | 2.7 |
| ARG370 | 2.8 | |||
| VAL243 | 2.4 | |||
| CHRITSC | −7.6 | 4 | VAL243 | 2.3 |
| ARG370 | 2.3 | |||
| SER447 | 2.8 | |||
| ARG457 | 2.8 | |||
| COUITSC | −7.2 | 2 | GLN549 | 2.5 |
| ARG370 | 2.6 | |||
| Darbufelone | −9.94 | 2 | ASN425 | 2.117 |
| HIS600 | 2.073 | |||
| PITSC | −5.6 | 1 | ARG370 | 2.6 |
| SFN | −4.1 | 2 | LEU448 | 3.4 |
| ARG457 | 2.7 |
BE binding energy (kcal/mol), H bond hydrogen bond
Anti-cancer activities
Effect of silencing CD44v6 on colon tumor cell proliferative response
It has been established that many cancer chemopreventive agents are known to lower cancer risk by suppressing HA/CD44-signaling pathway (Bourguignon et al., 2009; Cordo Russo et al., 2008), which subsequently leads to attenuation of pro-inflammatory mediators and their activities. Natural anti-oxidants like ITSCs found in cruciferous vegetables have been under investigation due to their potential anti-cancer effects. However, not many studies have been devoted to examining structure–activity relationships between ITSC structure and HA/CD44 activation as well as subsequent anti-inflammatory action. In the present work we have employed five common phytochemical motifs in conjunction with the thiosemicarbazone side chain containing isothiocyanate group as shown in Fig. 1 and in the Fig. 2.
Fig. 2.
a Docking figures of ITSC analogs in COX-2 protein cavity. b Docking figures of ITSC analogs in 5-LOX protein cavity
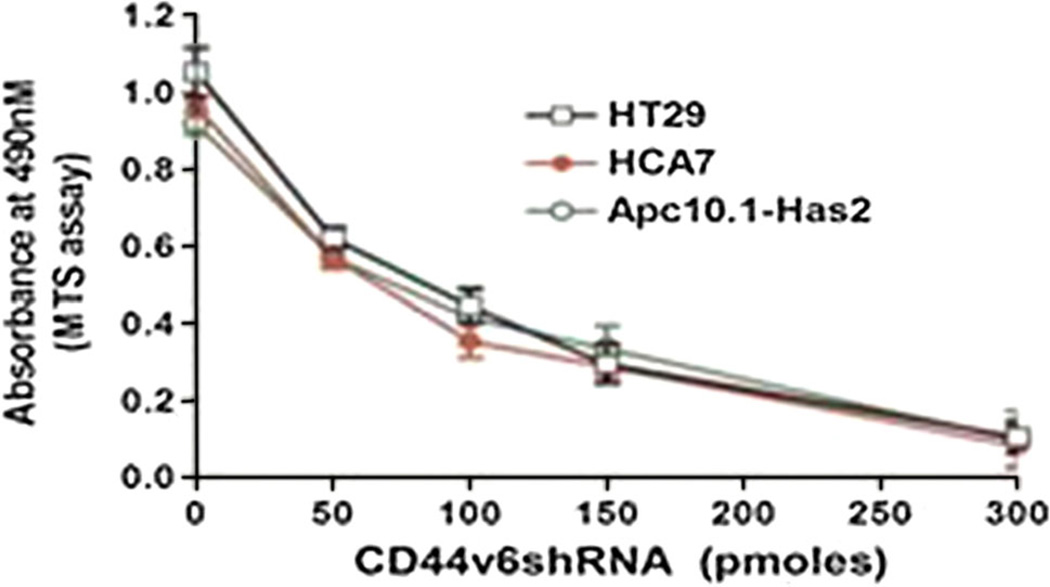
In order to ascertain whether HA-CD44v6-mediated signaling interaction with COX-2 and 5-LOX contributes to colon tumor cell survival which is the focus of present study, we determined the effect of CD44v6shRNA on colon tumor cell proliferation. The effect of silencing CD44v6 on colon tumor cell survival was studied in HCA7, HT29, and murine intestinal epithelial Apc10.1Has2 cells stably expressing Has2 cDNA (Fig. 3). It was observed that treatment of these colon tumor cells with CD44v6shRNA for 24 h followed by 48 h incubation significantly decreased cell proliferation, whereas treatment with the controlshRNA (scrambled shRNA) rendered cell proliferation unchanged (data not shown). The results in Fig. 3 also indicate that the order of inhibition of CD44v6shRNA in terms of IC50 values was: HCA7 (65 pmol) < Apc10.1Has2 (95 pmol) < HT29 (105 pmol), respectively. These results are in agreement with our previous findings that the HA-CD44v6-activated signaling is an important activator of oncogenic growth and chemo-resistance in colon cancer cells.
Fig. 3.
Effect of silencing CD44v6 on cell proliferation of colon tumor cells. HCA7, HT29, and Apc10.1-Has2 cells were seeded on a 96-well plate cultured in 100 µl medium. Each well was transfected with CD44v6shRNA at the indicated concentrations. After 24 h, 20 µl MTS solution was added. Cell viability was measured as absorbance at 490 nm. Likewise, these cells were transfected with scrambled shRNA (ControlshRNA), and the cell proliferation was measured as described before. ControlshRNA shows similar levels of cell proliferation with the various doses of controlshRNA treatment in HT29, HCA-7, and Apc10.1-Has2 cells (data not shown). The experiments were performed three times with identical outcome and the means ± SD are shown
Expression of CD44v6, COX-2 and 5-LOX proteins are suppressed in ITSC treated cells
It has been shown earlier that commonly available ITSCs such as SFN or PITSC can target COX-2 (Shan et al., 2009). Our recently published data have clearly shown that HA/CD44v6 signaling regulates COX-2 expression/activity both in vitro and in vivo (Misra et al., 2011). COX-2 also regulates HA/CD44 signaling (Misra et al., 2008a, b). These studies collectively suggest that expression of COX-2 in cancer cells is essential for cancer cell growth and that the function of ITSCs including SFN may involve engaging CD44 to target COX-2.
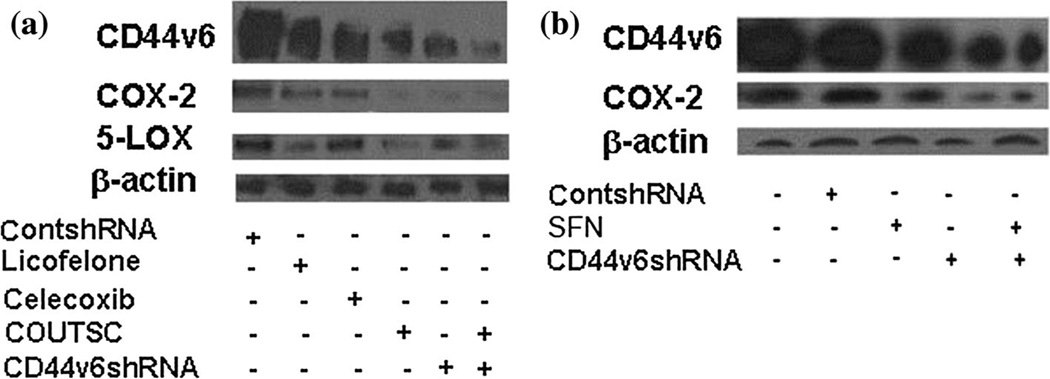
In the present study, we have found that a basal level of CD44v6, COX-2, and 5-LOX expression is present in human colon tumor HCA7 cells (Fig. 4a, b). However, cells pre-treated with CD44v6shRNA down-regulates COX-2 and 5-LOX expressions significantly when compared with the control-shRNA-transfected cells. Moreover, combining the ITSCs with CD44v6shRNA resulted in a pronounced decrease in basal CD44v6, COX-2, and 5-LOX expression. Thus, the reduction of COX-2 and 5-LOX expression (Fig. 4a, b) appears to be CD44v6-specific in colon cancer cells, and ITSCs engage CD44v6 to target COX-2 and 5-LOX. The order of inhibitory effect on COX-2, 5-LOX, and CD44v6 expression in colon tumor cells was found to be in the deceasing order of CD44 v6shRNA > COUITSC > Celecoxib > SFN > Licofelone, respectively. Interestingly, CD44v6 also seem to regulate 5-LOX in addition to COX-2 (Fig. 4a, b).
Fig. 4.
Inhibition of CD44v6 by ITSCs attenuates COX-2 and 5-LOX expression in colon tumor cells. HCA7 cells were either transfected with ControlshRNA or CD44v6shRNA for 24 h. Transfected cells were incubated for 48 h for growth and then treated with 16 h addition of IC50 concentrations (obtained from Table 2a, b) of Licofelone, Celecoxib, SFN, COUITSC, or their combination as indicated in the figure. Western blot analysis for CD44v6, COX-2, 5-LOX, and β-actin was then carried out. Data are representative of three independent experiments. Results indicate that COX-2 upregulation in colon tumor cells is dependent on the activation of CD44v6 and signaling, while ITSCs attenuates this signaling
Effect of synthesized analogs on proliferative activity of colon tumor cells
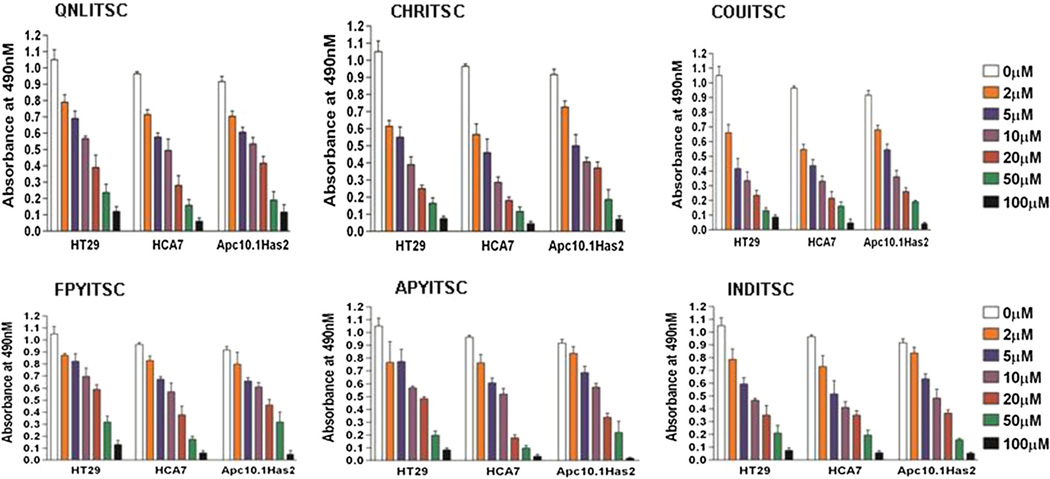
Since cellular ITSCs have been shown to target COX-2 and 5-LOX levels by the cellular HA-CD44v6 pathway, we analyzed cytotoxicity of these ITSC analogs in HT-29, HCA7 human colon cancer cells using the MTS assay. In order to examine the structural influence of the present ITSCs on the proliferation potential of HA-synthase 2 (Has2), we used the Apc10.1 murine intestinal epithelial cell line (De Giovanni et al., 2004), which is stably transfected with Has2 expression plasmid construct, as a screening model for HA. The HT-29, HCA7, and Apc10.1Has2 cells were treated with the indicated concentrations of ITSCs for 24 h and the effect of ITSC analogs on the MTS activity was compared (Fig. 5). A comparative account of the IC50 values of all compounds provided in Tables 3 and 4 revealed the following order in their IC50 values: COUITSC (2.8 µM) > CHRITSC (3.8 µM) > INDITSC (5.0 µM) > QNLITSC (6.8 µM) > APYITSC (12.0 µM) > FPYITSC (12.5 µM) (Fig. 5; Tables 3, 4) indicating COUTSC to be the most potent analog.
Fig. 5.
Anti-proliferative effect of synthesized ITSC analogs on colon cancer cells. HT29, HCA-7, and Apc10.1-Has2 cells (stable transfectant expressing Has2) were transfected with pSicoR scrambled shRNA (controlshRNA), pSicoR-CD44v6shRNA (CD44v6shRNA), were left untreated, treated with various doses of FPY TSC, APY TSC, IND TSC, CHR TSC, COU TSC, or QNL TSC. Cell proliferation was measured by an MTS assay and expressed as the mean absorbance at 490 nm/20 × 103 cells/90 min. The experiments were performed three times with identical outcome and the means ± SD are shown. Statistical analysis was done using analysis of variance (ANOVA) as applicable when compared between the various concentration groups of the analogs with untreated group. P ≤ .05 was considered statistically significant. In case of QNLITSC, CHRITSC, COUITSC, and INDITSC analogs when used for HT29 and HCA7 cells the results with 2–100 µM when compared to that of untreated group are statistically significant, but when FPYITSC and APYITSC analogs were used, the results with 5–100 µM when compared to that of untreated group are statistically significant. In case of Apc10.1Has2 when QNLITSC, CHRITSC, and COUITSC analogs were used the results with 2–100 µM are statistically significant when compared to that of untreated group, but when FPYITSC, APYITSC, and INDITSC analogs were used the results with 5–100 µM are statistically significant when compared to that of untreated group
Table 3.
Anti-proliferative potential of ITSC analogs
| Cell lines | FPYITSC IC50 (µM) |
APYITSC IC50 (µM) |
QNLITSC IC50 (µM) |
CHRITSC IC50 (µM) |
COUITSC IC50 (µM) |
SFN IC50 (µM) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Has2 | − | + | − | + | − | + | − | + | − | + | − | + |
| HT29 | 28.3 ± 2.9 | 55.7 ± 5.7 | 14.9 ± 1.7 | 42.6 ± 5.1 | 15.2 ± 1.9 | 5.4 ± 5.7 | 5.8 ± 1.2 | 21.4 ± 3.6 | 3.9 ± 0.9 | 12.3 ± 2.3 | 39.2 ± 5.9 | 52.8 ± 6.7 |
| HCA-7 | 12.5 ± 1.1 | 35.75 ± 2.9 | 12.0 ± 1.4 | 39.7 ± 4.9 | 12 ± 1.5 | 38.6 ± 3.9 | 3.8 ± 1.0 | 13.4 ± 1.5 | 2.8 ± 0.4 | 16.4 ± 1.1 | 35 ± 4.4 | 46.5 ± 8.5 |
| Apc10.1 | 8.9 ± 01.1 | 19.3 ± 1.7 | 5.39 ± 0.9 | 10.5 ± 1.3 | 12 ± 1.5 | 38.6 ± 3.9 | 2.9 ± 0.7 | 6.5 ± 2.5 | 2.3 ± 0.6 | 5.7 ± 1.3 | 29.4 ± 1.6 | 45.7 ± 5.8 |
Table 4.
Anti-proliferative potential of ITSC analogs
| Cell lines | INDITSC IC50 (µM) |
Phenyl ITSC (PEITSC) IC50 (µM) |
Celecoxib IC50 (µM) |
Licofelone IC50 (µM) |
CD44v6shRNA IC50 (pmoles) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Has2 | − | + | − | + | − | + | − | + | − | + |
| HT29 | 6.6 ± 7 | 24.0 ± 2.4 | 32.0 ± 4.9 | 52.6 ± 5.1 | 4.8 ± 1.6 | 13.5 ± 1.7 | 72.2 ± 6.9 | 123.7 ± 10.9 | 65.5 ± 6 | 169.6 ± 15 |
| HCA-7 | 5.0 ± 1.3 | 28.4 ± 1.9 | 28.0 ± 3.7 | 39.7 ± 4.9 | 3.8 ± 1.3 | 10.7 ± 1.5 | 58.9 ± 6.4 | 115.9 ± 9.5 | 58.2 ± 5 | 135.0 ± 11.6 |
| Apc10.1 | 4.2 ± 1.1 | 9.8 ± 19 | 19.5 ± 2.6 | 37.5 ± 3.3 | 2.8 ± 0.8 | 11.5 ± 0.8 | 41.5 ± 4.8 | 72.5 ± 7.9 | 45.8 ± 5.9 | 75.5 ± 8.9 |
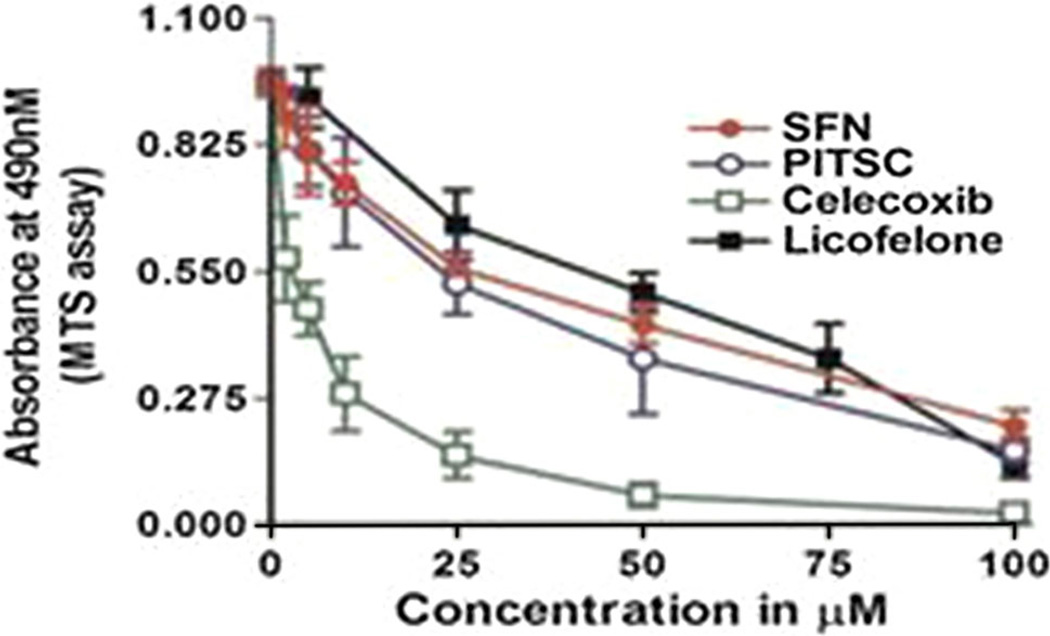
Additionally, we also determined the effect of SFN and standard compound PITSC as well as COX-2 specific inhibitor Celecoxib, and COX/LOX dual inhibitor Licofelone on the proliferation of HT29, HCA7, and Apc10.1-Has2 cells (Fig. 6). The order of inhibition (IC50) of these compounds was found to be: COUITSC (2.8 µM) > Celecoxib (3.8 µM) > PITSC (28 µM) > SFN (35 µM) > Licofelone (68 µM) (Figs. 5, 6; Tables 3, 4). These studies (Figs. 3–6) collectively indicate that apoptosis resistance of the colon tumor cells is most probably due to the enhanced expression of HA-CD44v6 interacting with COX-2 and 5-LOX target genes.
Fig. 6.
Anti-proliferative effect of standard ITSCs (SFN and PITSC), Celecoxib, and Licofelone on colon cancer cells. HCA-7 cells were left untreated, or treated with 16 h addition of various doses (as indicated in the figure) of Licofelone, Celecoxib, SFN, or PITSC for a period of 48 h. Cell proliferation was measured by an MTS assay and expressed as the mean absorbance at 490 nm/20 × 103 cells/90 min. The experiments were performed three times with identical outcome and the means ± SD are shown
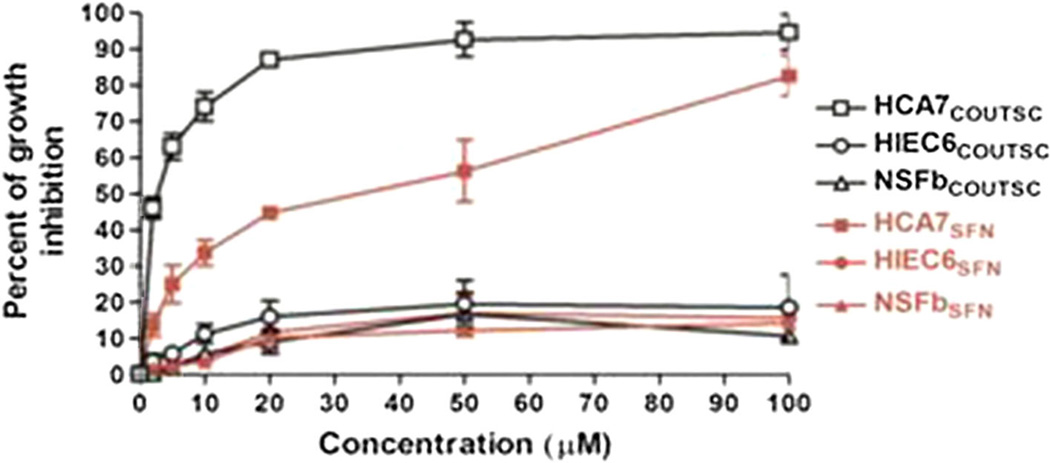
Effect of novel analogs on normal cells
In control experiments, we confirmed that the most potent analog COUITSC exhibited no pronounced cytotoxicity (as determined by MTT assay) to normal primary skin fibroblasts (NSFb) as well as to normal human intestinal epithelial cells (HIEC6) (Fig. 7). Similar results were also found in case of other ITSC analogs as well as CD44v6shRNA and Licofelone compounds (data not shown).
Fig. 7.
COUITSC exhibited no pronounced cytotoxicity to normal cells. CA-7 cells, human normal epithelial cells, and human normal skin fibroblasts were left untreated, or treated with 16 h addition of various doses (as indicated in the Fig. 7) of COUITSC and 24 h later mitochondrial activity/viability was measured by MTT assay. The experiments were performed three times with identical outcome and the means ± SD are shown
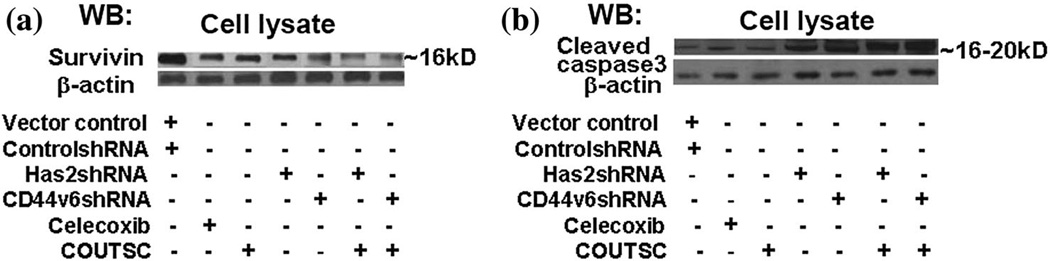
Effect of new ITSC analogs on survivin and caspase-3 proteins
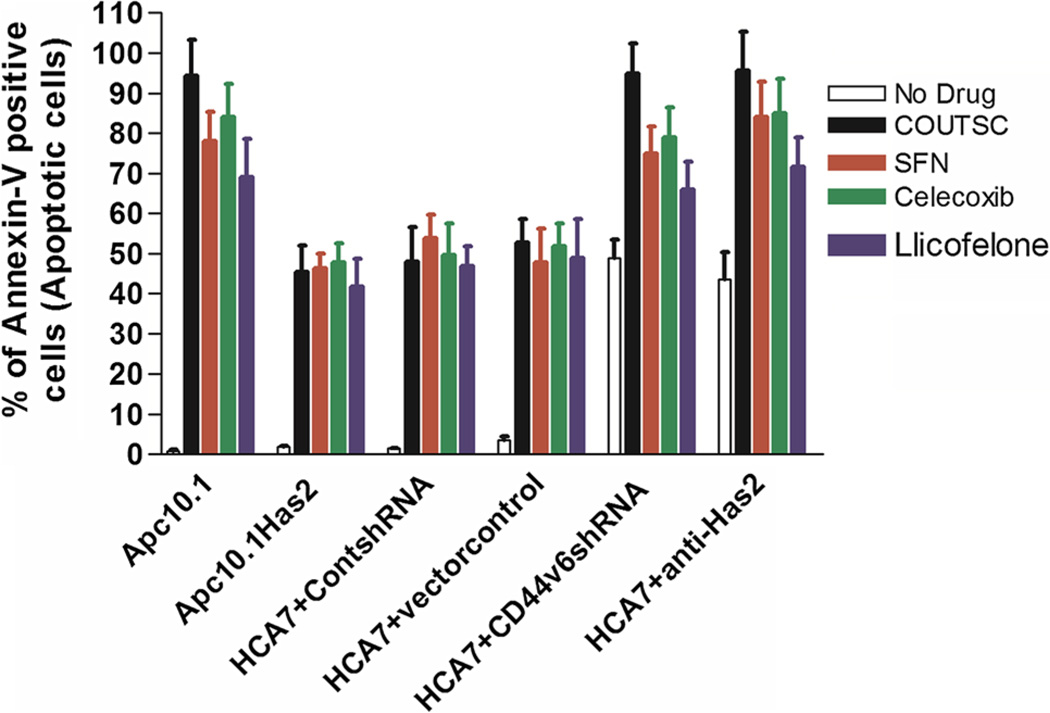
In order to determine the mechanistic details of these changes in the colon cancer cells proliferation, we performed tumor cell apoptosis assays using Apc10.1 (empty vector pCIneo transfected) and Apc10.1-Has2 cells which is stably transfected with Has-expression plasmid construct (to examine the effect of HA in the presence or absence of Has2) and controlshRNA, CD44v6shRNA-transfected HCA7, and HT29 cells (to examine the effect of silencing CD44v6 in the presence or absence of CD44v6shRNA), respectively. In the absence of Has2 expression, the Apc10.1 cells treated with COUITSC displayed an increase in apoptotic tumor cells with IC50 value of 2.3 µM (Table 3). Results in Fig. 8 indicate that the number of apoptotic cells (Annexin-V positive) was increased in the following order: COUITSC > Celecoxib > SFN > Licofelone. On the other hand, over-expression of Has2 or increased HA levels enhanced cell survival and reduced apoptosis in untreated controls (Tables 3, 4; Fig. 8). Furthermore, pre-treatment of HCA7 cells with CD44v6shRNA, or anti-Has2 alone significantly increased tumor cell apoptosis compared with controlshRNA, or vector control pre-treatment (Fig. 8) indicating that HA and CD44v6 are necessary for cell survival. Moreover, down-regulation of CD44v6 by CD44v6shRNA, or down-regulation of HA by anti-Has2 effectively enhanced drug sensitivity beyond the respective control-treated cells (Fig. 8, colored bars).
Fig. 8.
ITSC-induced Apoptosis in colon cancer cells Analysis of ITSC-induced Apoptosis in HCA7, Apc10.1, and Apc10.1Has2 cells. Here we designate the cells apoptotic when they display Annexin V-positive staining. In each sample, at least 1,000 cells from five different fields were counted. The percentage of apoptotic cells calculated as Annexin V-positive cells/total number of cells. The values are presented as the mean ± SD. Statistical analysis was done using analysis of variance (ANOVA) as applicable when compared between the groups. P ≤ .05 was considered statistically significant. When the group Apc10.1Has2 compared with Apc10.1, or when HCA7 + CD44v6shRNA compared to HCA7 + ControlshRNA, or HCA7 + anti-Has2 compared to HCA7 + vectorcontrol, results are considered statistically significant
These observations strongly suggest that HA is indeed responsible for the increase in tumor cell survival leading to the enhancement of chemo-resistance (Tables 3, 4 and Fig. 8).
Effect of ITSC on survivin, caspase-3, expression
To further assess whether the ITSCs effects on colon HCA7, HT29, and Apc10.1Has2 cells might affect colon tumor cell-specific behavior (e.g., chemo-resistance), we decided to analyze the expression of the chemo-resistance protein survivin and cleaved caspase-3. HA-CD44 interaction has been shown to induce the expression of survivin in tumor cells (Bourguignon et al., 2009). In addition, frequent co-expression of COX-2 and survivin was assessed by immuno-histochemistry in human lung cancer specimens of the patients revealed a marked reduction of tumor survivin levels in patients treated with COX-2 inhibitors prior to surgery (Krysan et al., 2004). The question of whether expression of survivin (induced by HA-CD44-COX-2 interaction signaling) is the target of ITSCs analogs has not been investigated systematically. To answer this question, immunoblot analyses using antibodies (e.g., anti-survivin antibody and β-tubulin) (as loading control antibody) were employed to detect the production of survivin, caspase-3, and β-tubulin in HCA7 cells. Our data indicated that the expression of survivin (Fig. 9) is significantly increased in HCA7 cells that are treated with CD44v6shRNA, COUITSC, Celecoxib, and their combination (Fig. 9) down-regulated survivin level. Likewise results from Fig. 9 indicated that cleaved caspase-3 levels were not detectable in non-apoptotic cells but significantly increased with the treatment of Cd44v6shRNA, COUITSC, or Celecoxib, or their combinations. These results indicate that HACD44v6-activated COX-2 signaling actively participate in the up-regulation surviving, and the data are consistent with previously reported data where COX-2 stabilizes survivin in cancer cells (Krysan et al., 2004).
Fig. 9.
ITSCs significantly decreased levels of chemo-resistant protein survivin, and increased apoptotic protein caspase-3 in HCA7 cells. Cell lysates isolated from HCA7 cells that express high level of HA, transfected with either vector control, or anti-Has2 cDNA, controlshRNA, CD44v6shRNAHA for 24 h, or treated with 16 h addition of celecoxib, or COUITSC, or first transfected with CD44v6shRNA followed by a 16 h addition of celecoxib, or COUITSC were processed for immunoblotting using anti-survivin antibody a, anti-caspase-3 antibody b, or anti-β-actin antibody (as a loading control). Data are representatives of three independent experiments
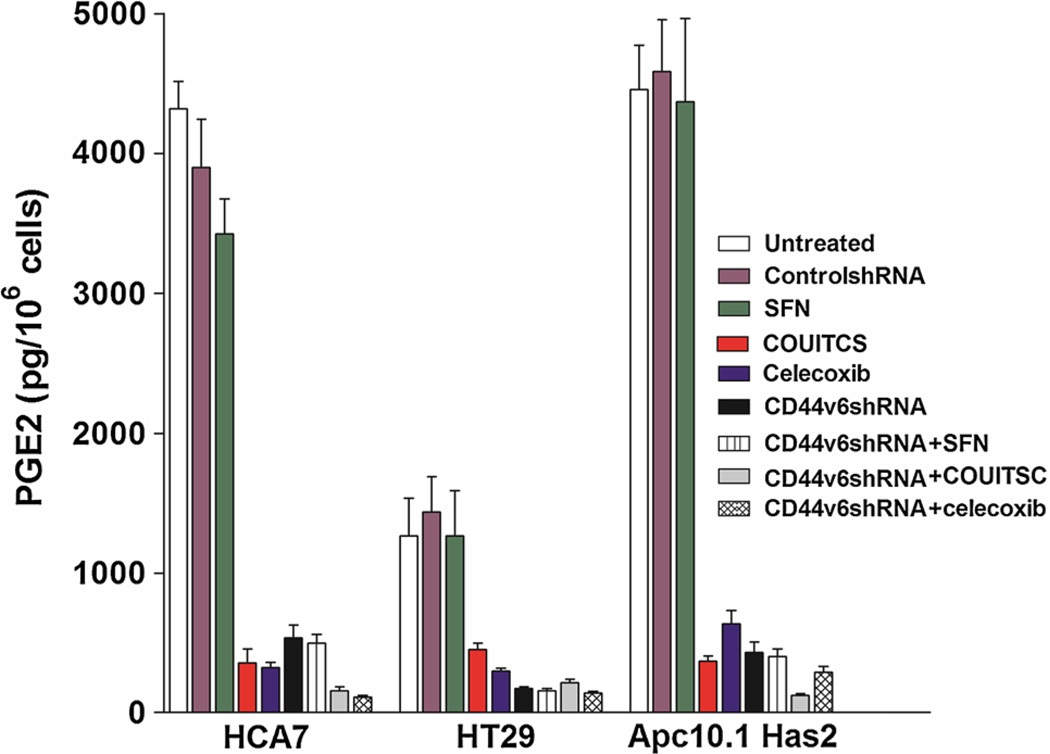
Effect of ITSC analogs on PGE2 synthesis
It is becoming clear that cancers are dependent upon a supportive inflammatory microenvironment, and that the cytokine prostaglandin E2 (PGE2) contributes to it and cancer progression (Park et al., 2006). The mechanism through which PGE2 contributes to tumor formation is partly through activation of HA-CD44 pathway. Similarly, the development of one significant cell type within the inflammatory environment is enhanced by production of prostaglandin E2 by tumor cells (Apte et al., 2006a, b; Krelin et al., 2007). These observations led us to develop the hypothesis that ITSCs may reduce the expression of PGE2. Subsequently we investigated whether anti-neoplastic effects of the present analogs as well as Celecoxib on CRC cells are mediated through the inhibition of PGE2 production. ELISA analyses were used to detect the levels of PGE2 concentration from the COX-2 positive (HCA7, HT29) and Apc10.1-Has2 cell lines (Patsos et al., 2010). The ELISA analyses revealed high levels of PGE2 in colon cancer cell line HCA7, followed by HT-29 (Fig. 10), which were dramatically decreased following the treatment with two potent ITC analogs, viz. CHRITSC and COUITSC, respectively. These analogs also substantially reduced the PGE2 production in COX-2 positive HCA7, HT29, and Apc 10.1 cells (Fig. 10) suggesting that suppression of cell survival as well as chemo-resistance (Figs. 3–9) by COUITSC may be due to the HA/CD44v6 interaction in these cells through COX-2/5-LOX-dependent mechanism (Figs. 3–11; Tables 3, 4).
Fig. 10.
Effect of ITSCs on PGE2 levels in colon cancer cells ITSCs target CD44v6/COX-2 to alter PGE2 level in colon tumor cells. Cell supernatants from HCA7, HT29, and Apc10.1Has2 cells that express high level of HA, transfected with either controlshRNA, or CD44v6shRNAHA for 24 h, or treated with 16 h addition of 25 µM SFN, or 2.5 µM COUTSC or 2.5 µM Celecoxib or first transfected with CD44v6shRNA followed by a 16 h addition of 25 µM SFN, or 2.5 µM COUTSC or 2.5 µM Celecoxib were processed for Enzyme-linked immunosorbent assay analysis (ELISA) analysis of the PGE2 level. Bars represent the means ± SE (n = 3) and the results were compared with untreated cells. Statistical analysis was done using analysis of variance (ANOVA) as applicable when compared between the groups. P ≤ .05 was considered statistically significant. When the groups (CD44v6shRNA, CD44v6shRNA + SFN, CD44v6shRNA + COUITSC, CD44v6shRNA + celecoxib) compared with the corresponding control group (ControlshRNA), results are considered statistically significant. When the groups (COUITSC, celecoxib) compared with the corresponding untreated control group, results are considered statistically significant
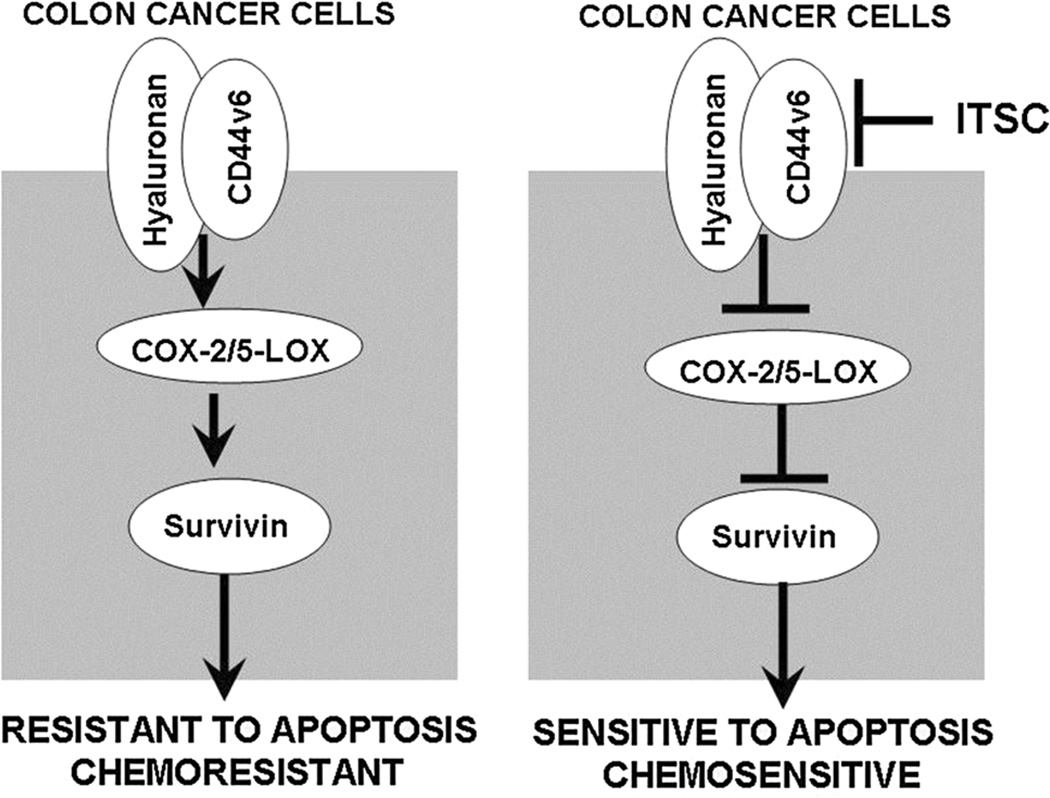
Fig. 11.
HA-CD44v6-COX-2/5-LOX pathway modulates resistance to apoptosis of colon cancer cells. Left panel In HA-CD44v6-COX-2-5-LOX overexpressing cells, elevated survivin and lower caspase3 levels accounts for the elevated apoptosis resistance of colon cancer cells. Right panel ITSCs suppress HA-CD44v6-COX-2-5-LOX pathway leading to increased caspase-3 and inhibition of survivin level, thus portraying cells more sensitive to apoptosis induction
Our present work thus indicates that among the new ITSC analogs seems to be a potent COX-LOX dual inhibitor which is active against colon cancer cells with IC50 values lower than the reference COX-2 specific drug, viz. Celecoxib. It inhibits PGE2 production in these cells indicating that the coumarin motif in it may have some special affinity toward CRC cells with respect to their COX-LOX status and which could be attributed to the presence of CD44v6 and HA expression in these cell lines (Kuhn et al., 2007). Interestingly, the standard inhibitor for HA-synthase is 4-Methylumbelliferone, which in fact is a coumarin compound. Although these compounds have been shown to inhibit hyaluronan synthase-2 (Has2) mRNA level and cell surface HA levels to some extent in other cell type (Vigetti et al., 2011; Lokeshwar et al., 2010), results of genetic knock-out studies show that deletion of Has2 and CD44 genes has very different outcomes. Has2-null mice die in utero due to impair development of cushion mesenchymes (Camenisch et al., 2000). On the other hand, CD44-null mice show increased inflammation because lack of CD44 impairs the clearing of HA from sites of inflammation (Teder et al., 2002). Thus, we think that CD44 may be a much safer therapeutic target than Has2.
Conclusions
Specifically, our findings indicate that HA-CD44v6-mediated COX-2/5-LOX signaling facilitates survivin production, which in turn, exerts its influence on colon tumor cell-specific functions including anti-apoptosis and chemo-resistance. This capacity of COX-2/5-LOX to enhance cell survival may help explain the COX-2-dependent resistance to radiation and chemotherapy in colon cancer. The work also demonstrates that over-expression of CD44v6shRNA as well as treatment with potent ITSC analog can significantly decrease the survival of colon tumor cells. Results described presently thus offer an opportunity to evolve potent inhibitors of HA synthesis and CD44v6 pathway and thus underscoring the importance of the ITSC analogs as chemopreventive agents for targeting HA/CD44v6 pathway.
Experimental Protocols
Materials
Apc 10.1, an intestinal cell line, was derived from Apc Min/+ mice and retains the host heterozygous Apc genotype. HCA7 clone 29 colon carcinoma cells were obtained from ECCA (United Kingdom). HT-29 cells were obtained from ATCC (Manassas, VA). RPMI, Dulbecco’s modified Eagle’s medium (DMEM) low glucose, glutamine, and pyruvate were from Life technology. Fetal bovine serum was from Atlanta Biological (GA); and l-glutamine, Gentamicin sulfate, and Amphotericin B were from Hyclone. HT29 cells were grown in RPMI media with 10 % FBS, and HCA7 and Apc10.1 cells were grown in RPMI media with 10 % FBS. Actinomycin D, cycloheximide, Nonidet P-40, ethylene glycol tetraacetic acid, sodium orthovanadate, glycerol, phenylmethylsulfonyl fluoride, leupeptin, pepstatin A, Aprotinin, and hydroxyethyl piperazine ethane sulfate (HEPES) were purchased from Sigma (St. Louis, MO). The celecoxib, licofelone, sulforaphane (SFN), phenyl isothiocyanate (PITSC), antibodies against CD44v6, COX-2, 5-LOX, caspase 3, β-actin, horse radish peroxidase-linked anti-rabbit and anti-mouse antibodies, and Luminol reagent were purchased from commercial sources (Sigma, Santa Cruz Biotechnology, Abcam, Ebioscience, Thermo Fischer). CD44v6shrNA or controlshRNA are designed as described in our earlier studies (Misra et al., 2009).
Synthetic procedures
All reagents for synthesizing ITSCs were from Sigma-Aldrich of analytical grade (AR) and were used without further purification. Solvents employed were purified by standard procedures prior to use (Perrin and Armarego, 1988). The ITSC analogs were synthesized by the condensation reaction between thiosemicarbazide and respective aldehydes/acetophenones in methanolic solvent following a protocol described earlier (Scovill et al., 1982; Adsule et al., 2006; Doyle et al., 1956; Kapoor et al., 2011; Li et al., 2010). The synthesized compounds complied with the analytical and spectroscopic data reported by these workers. The FT-IR spectra of the compounds were recorded on JASCO FTIR-4100 spectrophotometer. The MASS spectra of synthesized compounds were performed on Shimadzu GC-17A, GCMS-QP5050A version 1.10 Gas Chromatograph Mass Spectrometer by GC–MS analysis method at Department of Chemistry, University of Pune, India. 1H-NMR spectra were recorded on a FT-NMR Varian Mercury YH-300 Spectrometer in Pune University, India. The analysis of C, H, and N contents in the compounds was performed on HOSLI CHN analyzer in the Microanalytical laboratory at Department of Chemistry, University of Pune (INDIA).
Cell culture
Epithelial cell culture
HT29, HCA7, and Apc10.1Has2 were cultured in DMEM with normal glucose, glutamine, and pyruvate (Life technology) supplemented with 10 % fetal bovine serum, 2 µM l-glutamine, gentamicin sulfate (50 µg/ml), and amphotericin B (5 µg/ml) at 37 °C in 10 % CO2. The medium was changed every other day to remove dead and non-attached cells until colon cancer cells reached confluence. Monolayer cultures were maintained in the same medium. Colon cancer cells were used between the second and sixth passages in all experiments. All the treatments and transfection experiments were carried out in cells that were serum starved for 24 h.
Human Normal Epithelial Cells (HIEC6) and Human Normal Skin Fibroblasts (NSFbs) Cell Culture
The HIEC-6 human intestinal cell line (from J.F. Beaulieu, University of Sherbrook, Quebec, Canada) was maintained in DMEM (high glucose), 4 % fetal bovine serum, 20 mM HEPES buffer (pH 7.4), 50 units/ml penicillin, 50 µg/ml streptomycin, 10 µg/ml insulin, and 5 ng/ml human recombinant and used between the 15th and 17th passage in this study as described earlier (Misra et al., 2008a, b). Cell lines were grown at 37 °C in 5 % CO2.
The NSFbs were isolated and cultured as previously reported (Ghatak et al., 2013, 2014). Briefly, skin tissues were diced (approx. 0.5 mm × 0.5 mm pieces) and cultured in DMEM with normal glucose, glutamine, and pyruvate (Life technology) supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, gentamicin sulfate (50 µg/ml), and amphotericin B (5 µg/ml) at 37 °C in 10 % CO2. The medium was changed every 3 days to remove dead and non-attached cells until fibroblasts reached confluence. Monolayer cultures were maintained in the same medium. Skin fibroblasts were used between the second and fourth passages in all experiments.
Cell lysis and immunoblotting
Colon cancer cells were cultured until they were confluent. Cells were washed twice at 4 °C with PBS, harvested with 0.05 % Versene, and then washed in cold PBS again. The cells were pelleted by centrifugation at 5,000×g for 2 min at 4 °C. The pellet was treated with the lysis buffer containing 1 % Nonidet P-40, 0.5 mM EGTA, 5 mM sodium orthovanadate, 10 % (v/v) glycerol, 100 µg/ml phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin, 1 µg/ml pepstatin A, 1 µg/ml aprotinin, and 50 mM HEPES, pH 7.5. The lysates were clarified by centrifugation at 12,000×g for 10 min at 4 °C and then stored at −80 °C as described previously (Misra et al., 1999, 1998, 2006; 2008a, b; 2003, 2005). Cell lysates (normalized for protein concentration) were analyzed by immunoblotting as described previously (Misra et al., 2006; 2008a, b). The proteins on the blots were analyzed with antibodies for CD44v6, COX-2, 5-LOX, surviving, and 5-LOX (β-tubulin and β-actin as internal standards), and detected by luminol reagent (Santa Cruz Biotechnology, CA) following treatment with horse radish peroxidase-linked anti-rabbit or anti-mouse antibody as secondary. Each protein was analyzed in samples from at least three independent experiments from each set of fibroblasts.
RNA Silencing
ControlsiRNA (scrambledsiRNA) and CD44v6shRNA were prepared as described previously (Cheng et al., 2006; Misra et al., 2009).
CD44v6shRNA Cloning in pSicoR Vectors
Double-stranded oligonucleotide cassettes for control-shRNA (scrambledshRNA), CD44v6shRNA were prepared. The linearized pSicoR vectors were ligated to the double-stranded oligonucleotide cassettes. The resulting pSicoR-CD44v6shRNA (CD44v6shRNA) transfectants constitutively silence CD44v6 genes in the cells. pSicoR-scrambledshRNA (controlshRNA) transfectants were used as control to the above shRNA transfectants.
Transient transfection using colon cancer cells
All transfections were done using Lipofectamine (Invitrogen) in cultures at ~75 % confluence. After transfection, the cultures were grown for another 72–96 h for analyses.
Determination of PGE2 in the culture medium
Colon cancer cells (1 × 105 cells/well) were cultured in 6-well culture plates with appropriate media plus 10 % FBS. At confluence, culture medium was discarded, and each well was washed with PBS twice. Serum-free medium (QBSF-51; Sigma) was then added followed by incubation for various time points. The supernatants were collected and stored at −80 °C until use. PGE2 concentrations in culture supernatants were measured by an ELISA assay from (Elisa kit was from Cayman Chemicals).
Cell Proliferation Assay
Cell proliferation was measured by CellTiter 96® AQueous Assay (Promega). The reagent consists of solutions of a novel tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS) and an electron coupling reagent (phenazine methosulfate; PMS). MTS is reduced by cells into a soluble formazan product in tissue culture medium. Growing cells were harvested, counted, and seeded at the 20 × 103 cells (200-µl volume) into 96-well microplates. After 18 h, culture medium was replaced by medium containing experimental agents (200 µl) as indicated in Fig. 2. MTS/PMS solution was freshly prepared at 2/0.92 mg/ml in DPBS. Ten micro liters of MTS/PMS reagent (excess MTS/PMS) was added to each well and then incubated at 5 % CO2 atmosphere. Absorbance at 490 nm was recorded at 90 min. A blank experiment detecting cell-free background absorbance was also performed in parallel. Absorbance shown in the figures was obtained by subtracting the absorbance of cell-free equivalents. Trypan blue exclusion showed less than 1 % cell death both before and after the assays.
Cytotoxicity analysis
MTT was first prepared as a stock solution of 5 mg/ml in phosphate buffer (PBS, pH 7.2) and was filtered. At the end of the treatment period of our tested compounds (24 h), with four different concentrations in triplicate, 25 µl of MTT solution was added to each well. After incubation for 4 h at 37 °C, 100 µl of solubilizing buffer (10 % sodium dodecyl sulfate dissolved in 0.01 N HCl) was added to each well. After overnight incubation, the 96-well plate was read by an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm for absorbance density values to determine the cell viability. The viable cells produced a dark blue formazan product, whereas no such staining was formed in the dead cells.
IC50 of ITSC analogs, CD44v6shRNA, Celcoxib, Licofelone, SFN, and PITSC in colon cancer cell growth
Colon cancer cells were either untreated or treated with ITSCs, ITSC analogs, or celecoxib, or licofelone, or SFN, and PITSC, in the presence or absence of HA, or controlshRNA, or CD44v6shRNA as above. These cells were then plated in 24-well culture plates in 0.2 ml of DMEM, or RPMI medium (chosen appropriately for each cell line) supplement (Invitrogen) containing no serum for 24 h at 37 °C in 5 % CO2, 95 % air. In each experiment, a total of five plates (5wells/treatment)were used. Experiments were repeated 5 times. The in vitro growth of these cells was determined by measuring increases in cell number using Coulter counter. IC50 is designated as the concentration (nM) of chemotherapeutic drugs (e.g., ITSC analogs, CD44v6shRNA, celcoxib, licofelone, SFN, or PITSC) that causes 50 % inhibition of tumor cell growth. IC50 values are presented as the means ± SD. All assays consisted of at least 5 replicates and were performed on at least five different experiments analysis.
Analyses of ITSC analogs-induced apoptosis in colon cancer cells
Cells were designated apoptotic when displaying Annexin V-positive staining. In each sample, at least 1,000 cells from five different fields were counted, with the percentage of apoptotic cells calculated as Annexin V-positive cells/total number of cells (Annexin V-positive cells/total cells × 100 %). The values are presented as the mean ± SD.
Statistical analysis
Each experiment was repeated three times for each set of fibroblasts, which were considered as n = 9 and pooled for statistical analysis. Western blot analyses, proliferation experiments (MTS assay), and cytotoxic assay (MTT assay) for each separate experiment were repeated between 3 and 4 times, depending upon the particular study. Data are expressed as ±SD. Statistical analysis of the Western blots was performed using t test with Mann–Whitney modification or analysis of variance (ANOVA) as applicable. P ≤ .05 was considered statistically significant.
Acknowledgments
This work was supported by 1R03CA167722-01A1 (to S. M. and S. G.), P20RR021949 (to S. G.), P20RR016434 (to S. M., S. G., and R. R. M.), P20RR16461-05 (to S. G., and R. R. M.), HL033756-24, 1 P30AR050953 (to V. C. H.), EPS 0903795 (to S. M.), and MCRC 39919 (to S. G. and S. M.). S. P. acknowledges Mr. P. A. Inamdar and Dr. E. M. Khan for their keen interest and encouragement. We thank Dr. Carla De Giovanni (University of Bologna, Bologna, Italy) for Apc10.1 cells.
Contributor Information
Suniti Misra, Regenerative Medicine and Cell Biology, Medical University of South Carolina, Charleston, SC 29425, USA.
Shibnath Ghatak, Email: ghatak@musc.edu, Regenerative Medicine and Cell Biology, Medical University of South Carolina, Charleston, SC 29425, USA.
Alok Vyas, ISTRA, Department of Chemistry, Abeda Inamdar College, University of Pune, Pune 411001, India; Department of Bioinformatics and Computer Science, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth, Tathawade, Pune 411033, India.
Paul O’Brien, Hematology/Oncology Division, Medical University of South Carolina, Charleston, SC 29425, USA.
Roger R. Markwald, Regenerative Medicine and Cell Biology, Medical University of South Carolina, Charleston, SC 29425, USA
Madhukar Khetmalas, Department of Bioinformatics and Computer Science, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth, Tathawade, Pune 411033, India.
Vincent C. Hascall, Department of Biomedical Engineering/ND20, The Cleveland Clinic Foundation, Cleveland, OH, USA
James B. McCarthy, Department of Laboratory Medicine and Pathology, University of Minnesota Masonic Cancer Center, Minneapolis, MN, USA
Nikos K. Karamanos, Laboratory of Biochemistry, Department of Chemistry, University of Patras, Patras, Greece
Markku I. Tammi, University of Eastern Finland, P.O. Box 1627, 70211 Kuopio, Finland
Raija H. Tammi, University of Eastern Finland, P.O. Box 1627, 70211 Kuopio, Finland
Glenn D. Prestwitch, Department of Medicinal Chemistry, University of Utah, Salt Lake City, UT, USA
Subhash Padhye, Email: sbpadhye@hotmail.com, ISTRA, Department of Chemistry, Abeda Inamdar College, University of Pune, Pune 411001, India.
References
- Adsule S, Barve V, Chen D, Ahmed F, Dou QP, Padhye S, Sarkar FH. Novel Schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J Med Chem. 2006;49:7242–7246. doi: 10.1021/jm060712l. [DOI] [PubMed] [Google Scholar]
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumourigenesis, tumour invasiveness, metastasis and tumour-host interactions. Cancer Metastasis Rev. 2006a;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, Segal S, Voronov E. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumor invasiveness and tumour-host interactions. Eur J Cancer. 2006b;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B, Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, Hess TM, Woloj GM, Boisserie F, Anderson WF, Viner JL, Bagheri D, Burn J, Chung DC, Dewar T, Foley TR, Hoffman N, Macrae F, Pruitt RE, Saltzman JR, Salzberg B, Sylwestrowicz T, Gordon GB, Hawk ET. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Bhamre S, Sahoo D, Tibshirani R, Dill DL, Brooks JD. Temporal changes in gene expression induced by sulforaphane in human prostate cancer cells. Prostate. 2009;69:181–190. doi: 10.1002/pros.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumour suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumour cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Tang WY, Hsu CW, Tsai YT, Wu JF, Lin CW, Cheng YM, Hsu YC. Apoptosis induction in primary human colorectal cancer cell lines and retarded tumour growth in SCID mice by sulforaphane. Evid Based Complem Altern Med. 2012 doi: 10.1155/2012/415231. 415231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo Russo RI, Garcia MG, Alaniz L, Blanco G, Alvarez E, Hajos SE. Hyaluronan oligosaccharides sensitize lymphoma resistant cell lines to vincristine by modulating P-glycoprotein activity and PI3K/Akt pathway. Int J Cancer. 2008;122:1012–1018. doi: 10.1002/ijc.23122. [DOI] [PubMed] [Google Scholar]
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Damm S, Koefinger P, Stefan M, Wels C, Mehes G, Richtig E, Kerl H, Otte M, Schaider H. HGF-promoted motility in primary human melanocytes depends on CD44v6 regulated via NF-kappa B, Egr-1, and C/EBP-beta. J Invest Dermatol. 2010;130:1893–1903. doi: 10.1038/jid.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood RH, Ho E. Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr Rev. 2008;66:S36–S38. doi: 10.1111/j.1753-4887.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- De Giovanni C, Landuzzi L, Nicoletti G, Astolfi A, Croci S, Micaroni M, Nanni P, Lollini PL. Apc10.1: an ApcMin/+ intestinal cell line with retention of heterozygosity. Int J Cancer. 2004;109:200–206. doi: 10.1002/ijc.11690. [DOI] [PubMed] [Google Scholar]
- Doyle FP, Ferrier W, Holland DO, Mehta MD, Nayler JHC. Potential antituberculosis agents of the indole series. J Chem Soc. 1956:2853–2857. [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumours. Proc Natl Acad Sci USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Hascall VC, Berger FG, Penas MM, Davis C, Jabari E, He X, Norris JS, Dang Y, Markwald RR, Misra S. Tissue-specific shRNA delivery: a novel approach for gene therapy in cancer. Connect Tissue Res. 2008;49:265–269. doi: 10.1080/03008200802147845. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Hascall VC, Markwald RR, Misra S. Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem. 2010;285:19821–19832. doi: 10.1074/jbc.M110.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Bogatkevich GS, Atnelishvili I, Akter T, Feghali-Bostwick C, Hoffman S, Fresco VM, Fuchs JC, Visconti RP, Markwald RR, Padhye SB, Silver RM, Hascall VC, Misra S. Overexpression of c-Met and CD44v6 receptors contributes to autocrine TGF β1 signaling in interstitial lung disease. J Biol Chem. 2013 doi: 10.1074/jbc.M113.505065. 2013 Dec 9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S, Misra S, Norris RA, Rodrigue RM, Hoffman S, Levine RA, Hascall VC, Markwald RR. Periostin induces intracellular cross talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem. 2014 doi: 10.1074/jbc.M113.539882. 2014 Jan 27. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR, Newcomer ME. The structure of human 5-lipoxygenase. Science. 2011;331:217–219. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Kenney PM, Wang M, Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumourigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- Kapoor P, Fahmi N, Singh RV. Microwave assisted synthesis, spectroscopic, electrochemical and DNA cleavage studies of lanthanide(III) complexes with coumarin based imines. Spectrochim Acta A. 2011;83:74–81. doi: 10.1016/j.saa.2011.07.054. [DOI] [PubMed] [Google Scholar]
- Kassie F, Uhl M, Rabot S, Grasl-Kraupp B, Verkerk R, Kundi M, Chabicovsky M, Schulte-Hermann R, Knasmuller S. Chemoprevention of 2-amino-3-methylimidazo[4,5-f] quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis. 2003;24:255–261. doi: 10.1093/carcin/24.2.255. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, Apte RN. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumours. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- Krysan K, Merchant FH, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc’h N, Pold M, Seligson D, Chia D, Goodglick L, Wang H, Strieter R, Sharma S, Dubinett S. COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2004;18:206–208. doi: 10.1096/fj.03-0369fje. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, Zöller M. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–567. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- Lamy E, Scholtes C, Herz C, Mersch-Sundermann V. Pharmacokinetics and pharmacodynamics of isothiocyanates. Drug Metab Rev. 2011;43:387–407. doi: 10.3109/03602532.2011.569551. [DOI] [PubMed] [Google Scholar]
- Laskowski RA. PDBsum new things. Nucleic Acids Res. 2009;37:D355–D359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Hyman R. CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol. 1992;22:2719–2723. doi: 10.1002/eji.1830221036. [DOI] [PubMed] [Google Scholar]
- Lesley J, English N, Charles C, Hyman R. CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol. 2000a;30:245–253. doi: 10.1002/eji.1830221036. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000b;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang ZY, Wu JC. Synthesis, crystal structures, biological activities and fluorescence studies of transition metal complexes with 3-carbaldehyde chromone thiosemicarbazone. Eur J Med Chem. 2010;45:5692–5701. doi: 10.1016/j.ejmech.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Schwartz SJ, Sun D. Sulforaphane potentiates the efficacy of 17-allylamino 17-demethoxygeldanamycin against pancreatic cancer through enhanced abrogation of Hsp90 chaperone function. Nutr Cancer. 2011;63:1151–1159. doi: 10.1080/01635581.2011.596645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Prostaglandin H synthase 2 variant (Val511Ala) in African Americans may reduce the risk for colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 1998;7:647–652. [Google Scholar]
- Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N, Altman N. Antitumour activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613–2623. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Gatmaitan Z, Varticovski L, Arias IM. The role of phosphoinositide 3-Kinase in taurocholate-induced Trafficking of ATP-dependent canalicular transporters in rat liver. J Biol Chem. 1998;273:26638–26644. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Varticovski L, Arias IM. Phosphoinositide 3-kinase lipid products regulate ATP-dependent transport by sister of P-glycoprotein and multidrug resistance associated protein 2 in bile canalicular membrane vesicles. Proc Natl Acad Sci USA. 1999;96:5814–5819. doi: 10.1073/pnas.96.10.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–20315. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- Misra S, Hascall VC, Berger FG, Markwald RR, Ghatak S. Hyaluronan, CD44, and cyclooxygenase-2 in colon cancer. Connect Tissue Res. 2008a;49:219–224. doi: 10.1080/03008200802143356. [DOI] [PubMed] [Google Scholar]
- Misra S, Obeid LM, Hannun YA, Minamisawa S, Berger FG, Markwald RR, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem. 2008b;283:14335–14344. doi: 10.1074/jbc.M703811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Hascall VC, De Giovanni C, Markwald RR, Ghatak S. Delivery of CD44 shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICE. J Biol Chem. 2009;284:12432–12446. doi: 10.1074/jbc.M806772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1429–1443. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Ghatak S, Patil N, Dandawate P, Ambike V, Adsule S, Unni D, Venkateswara Swamy K, Padhye S. Novel dual cyclooxygenase and lipoxygenase inhibitors targeting hyaluronan-CD44v6 pathway and inducing cytotoxicity in colon cancer cells. Bioorg Med Chem. 2013;21:2551–2559. doi: 10.1016/j.bmc.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Patsos HA, Greenhough A, Hicks DJ, Al Kharusi M, Collard TJ, Lane JD, Paraskeva C, Williams AC. The endogenous cannabinoid, anandamide, induces COX-2-dependent cell death in apoptosis-resistant colon cancer cells. Int J Oncol. 2010;37:187–193. doi: 10.3892/ijo_00000666. [DOI] [PubMed] [Google Scholar]
- Perrin D, Armarego W. Purification of laboratory chemicals. New York: Pergamon Press; 1988. [Google Scholar]
- Reddy BS, Rao CV. Novel approaches for colon cancer prevention by cyclooxygenase-2 inhibitors. J Environ Pathol Toxicol Oncol. 2002;21:155–164. [PubMed] [Google Scholar]
- Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, Seibert K, Rao CV. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61. [PubMed] [Google Scholar]
- Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Scovill JP, Klayman DL, Franchino CF. 2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agents. J Med Chem. 1982;25:1261–1264. doi: 10.1021/jm00352a036. [DOI] [PubMed] [Google Scholar]
- Shan Y, Wu K, Wang W, Wang S, Lin N, Zhao R, Cassidy A, Bao Y. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells. Int J Oncol. 2009;34:1129–1134. doi: 10.3892/ijo_00000240. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Soumaoro LT, Iida S, Uetake H, Ishiguro M, Takagi Y, Higuchi T, Yasuno M, Enomoto M, Sugihara K. Expression of 5-lipoxygenase in human colorectal cancer. World J Gastroenterol. 2006;12:6355–6360. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer I. Epidemiology. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- Swamy MV, Herzog CR, Rao CV. Inhibition of COX-2 in colon cancer cell lines by celecoxib increases the nuclear localization of active p53. Cancer Res. 2003;63:5239–5242. [PubMed] [Google Scholar]
- Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- Telang U, Brazeau DA, Morris ME. Comparison of the effects of phenethyl isothiocyanate and sulforaphane on gene expression in breast cancer and normal mammary epithelial cells. Exp Biol Med (Maywood) 2009;234:287–295. doi: 10.3181/0808-RM-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Van Eylen D, Oey I, Hendrickx M, Van Loey A. Kinetics of the stability of broccoli (Brassica oleracea Cv. Italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. J Agric Food Chem. 2007;55:2163–2170. doi: 10.1021/jf062630b. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Rizzi M, Moretto P, Deleonibus S, Dreyfuss JM, Karousou E, Viola M, Clerici M, Hascall VC, Ramoni MF, De Luca G, Passi A. Glycosaminoglycans and glucose prevent apoptosis in 4-methylumbelliferone-treated human aortic smooth muscle cells. J Biol Chem. 2011;286:34497–34503. doi: 10.1074/jbc.M111.266312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Itano N, Narimatsu H, Kudo T, Morozumi K, Hirohashi S, Ochiai A, Ueda M, Kimata K. Elevated transcript level of hyaluronan synthase1 gene correlates with poor prognosis of human colon cancer. Clin Exp Metastasis. 2004;21:57–63. doi: 10.1023/b:clin.0000017203.71293.e0. [DOI] [PubMed] [Google Scholar]
- Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]