Figure 6.
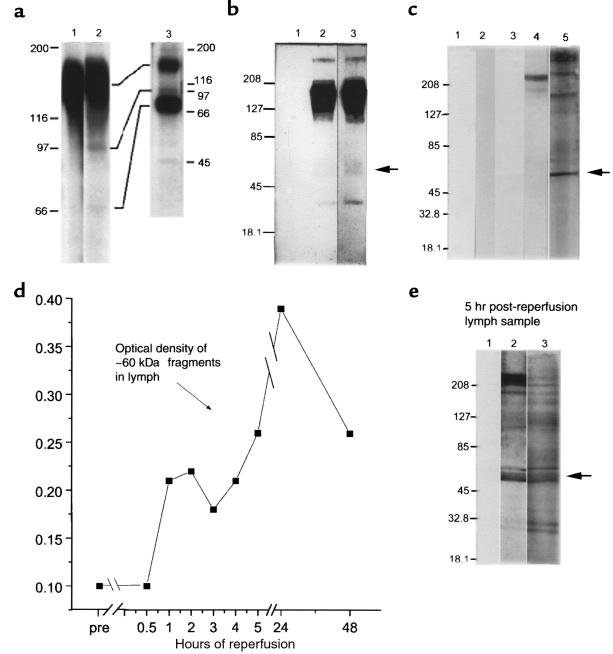
Fragmentation of cell-surface VLA-5 after exposure to FN120. (a) Lysates of U937 cells, surface labeled with 125I, were immunoprecipitated by affinity-purified antibodies to α5 and then fractionated by SDS-PAGE before radioautography. Lane 1, untreated cells; lane 2, FN120-treated cells; and lane 3, anti-α5 immunoprecipitates of culture supernatants of radiolabeled cells, previously exposed to FN120, demonstrating the release of α5 fragments. (b) Lysates of U937 cells were immunoprecipitated with rabbit antibody to β1, fractionated, blotted to nitrocellulose, and then probed with affinity-purified rabbit antibodies to the cytoplasmic domain of α5 (lanes 2 and 3). Lane 1, lysates incubated only with the secondary antibody; lane 2, proteins from untreated cells; lane 3, proteins from FN120-treated cells in lanes 1 and 3. An arrow indicates that the 60-kDa fragment was more prominent after FN120 treatment. (c) U937 cells cultured with or without FN120. Lanes 1 and 3, supernatants from untreated cells; lanes 2, 4, and 5, supernatants from FN120-treated cells. Immunoblots probed with second antibody only (lanes 1 and 2), with anti-β1 antibodies (lanes 3 and 4), or with antibodies to the cytoplasmic domain of α5 (lane 5). Note the 60-kDa fragment identified with anti-α5 antibodies. (d) Serial densitometric measurements of a 60-kDa α5 fragment in immunoblots of cardiac lymph collected at intervals before occlusion and for 48 hours after reperfusion in a dog with significant myocardial ischemia. (e) Immunoblot of cardiac lymph collected 5 hours after reperfusion of ischemic myocardium. Lane 1 was developed with only second antibody; lane 2 with anti-β1; and lane 3 with antibodies to the cytoplasmic domain of α5. The arrow beside lane 3 indicates a prominent 60-kDa fragment detected by anti-α5 antibodies.