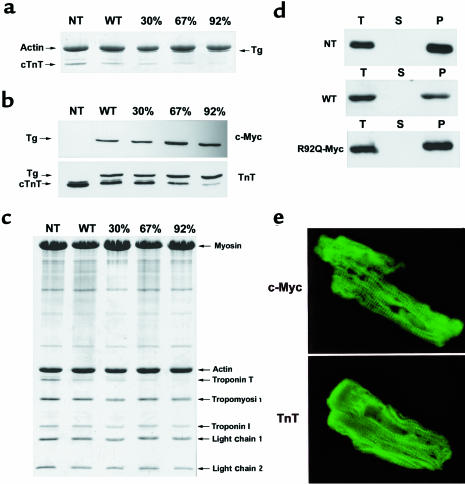
Figure 2.
Expression of WT-Myc and R92Q-Myc proteins in cardiac tissue. (a) Coomassie-stained SDS-PAGE gel of myofibrils isolated from non-Tg (NT), WT-Myc (WT), and 3 independent R92Q-Myc lines. A total of 5 μg myofibril protein was loaded per lane. Addition of the c-myc epitope tag decreases the mobility of the Tg protein (Tg) and allows unambiguous identification. Percentages (30%, 67%, 92%) represent Tg/endogenous cTnT ratios for the 3 R92Q-Myc lines. (b) Western blot analysis of myofibrils subjected to the same SDS-PAGE conditions as noted in a. Identical blots were probed with either a c-myc or cTnT mAb as indicated. The cTnT MAb detects both Tg and endogenous cTnT, and their relative positions are marked by arrows. The additional band immediately above the cTnT band (seen best in the NT lane) represents a known murine cTnT isoform. Note the progressive decrease in endogenous cTnT protein amounts among the 3 R92Q-Myc lines. (c) Myofibrils were purified from mouse hearts, subjected to SDS-PAGE, and Coomassie stained. Myofibrillar stoichiometry is maintained in all Tg lines. (d) Fractionation of cTnT. Three separate fractions were analyzed (T = total, S = supernatant, and P = pellet). Immunoblots of fractions from non-Tg (NT), WT-Myc (WT), and R92Q-67% (R92Q-Myc) were loaded for equal signal intensity and probed with either a cTnT (non-Tg) or c-myc (WT-Myc and R92Q-Myc) mAb as indicated. No Tg protein was detected in the S fraction for either WT-Myc or R92Q-Myc. (e) Transgene protein incorporation. Shown are confocal images of isolated adult cardiac myocytes probed with either c-myc (top; R92Q-67%) or TnT (bottom; non-Tg) mAb. ×4,300.