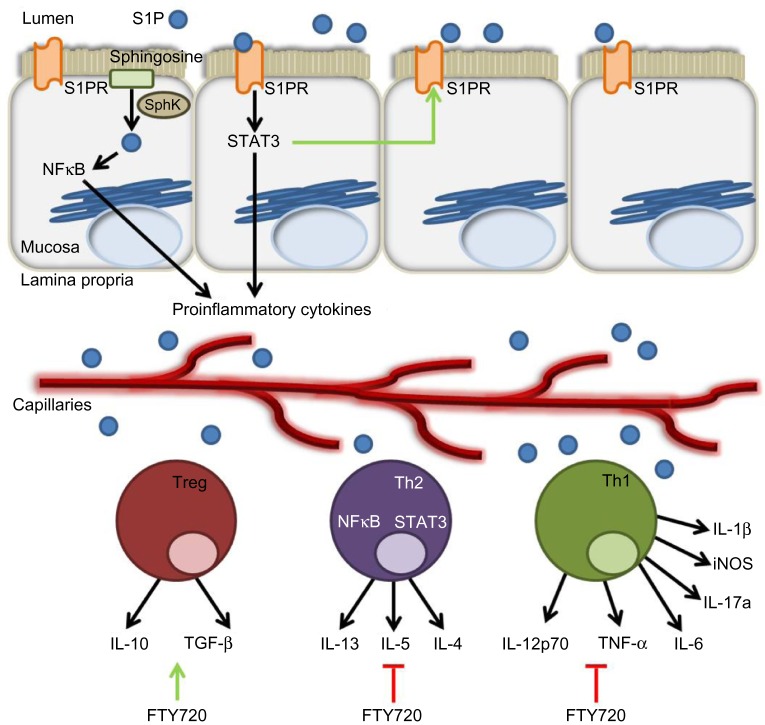
Figure 3.
The role of the S1P axis in adaptive immunity.
Notes: Dietary or bacterial S1P from the intestinal lumen bind to S1PRs and activate the STAT3 pathway in intestinal epithelial cells. Mucosal NFκB is activated by intracellular S1P produced by the phosphorylation of sphingosine by SphK. These two transcription factors transactivate numerous proinflammatory cytokine genes, thereby contributing to the recruitment of immune cells to the inflamed site. Moreover, STAT3 can amplify S1P/S1PR signaling by increasing the expression of S1PR1. Adaptive immune cells, namely Treg, Th1, and Th2 cells, produce a variety of cytokines in response to intestinal inflammation. Most notably, in response to NFκB and STAT3 pathway activation, Th1 and Th2 cells can produce an array of proinflammatory cytokines, including IL-1β, TNF-α, IL-5, and so on. The S1PR1 superagonist FTY720 increases Treg production of IL-10 and TGF-β, but inhibits proinflammatory cytokine production by Th1 and Th2 cells, possibly by acting through S1PRs.
Abbreviations: S1P, sphingosine-1-phosphate; S1PR, sphingosine-1-phosphate receptor; SphK, sphingosine kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; STAT3, signal transducer and activator of transcription 3; Treg, regulatory T-cells; IL, interleukin; Th, T-helper; iNOS, inducible nitric oxide synthase; TNF, tumor necrosis factor; TGF, transforming growth factor.