Fig. 1.
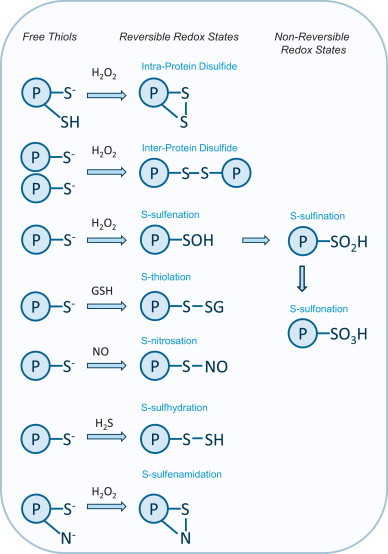
Summary of the oxidative modifications formed in protein thiols. Protein thiols can form a variety of oxidative modifications, including reversible (intra-protein disulfides, inter-protein disulfides, S-sulfenation, S-nitrosation, S-thiolation, S-sulfhydration, S-sulfenamidation) and non-reversible hyper-oxidized (S-sulfination , S-sulfonation) redox states. Some redox states, such as S-sulfenation, S-nitrosation or S-sulfhydration, can be intermediates that transition to disulfides. Prolonged exposure to oxidants can result in irreversible modifications such as S-sulfination or S-sulfonation.
