Figure A1.
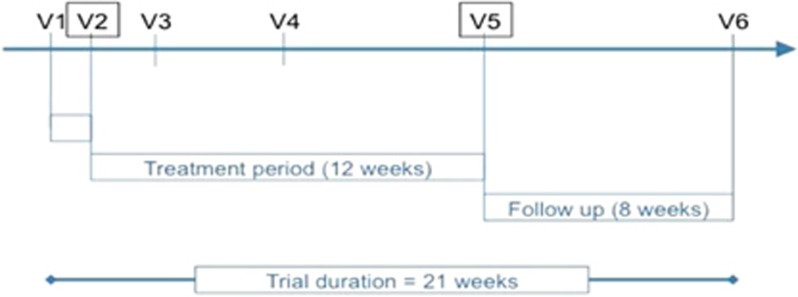
The course of the trial. Patients were screened for inclusion and exclusion criteria (see legend of Figure 1) at V1. If patients still fulfilled the study criteria at baseline (V2, 1 week after V1), they were then randomized into the study and received their first study medication at the study center after baseline investigations (interview, physical examination, sigmoidoscopy/colonoscopy, lab tests). At the interim visits 2 and 6 weeks after baseline (V3 and V4w), the Simple Clinical Colitis Activity Index (SCCAI), possible disease exacerbations, changes in medication, and adverse events (AEs) were assessed. The treatment period ended 12 weeks after baseline at V5 that involved the final study assessment (interview, physical examination, lab test, sigmoidoscopy). Responders of all study arms entered a 8-week follow-up period without study medication; those patients were asked to continue their comedication as taken before, unless they relapsed.
