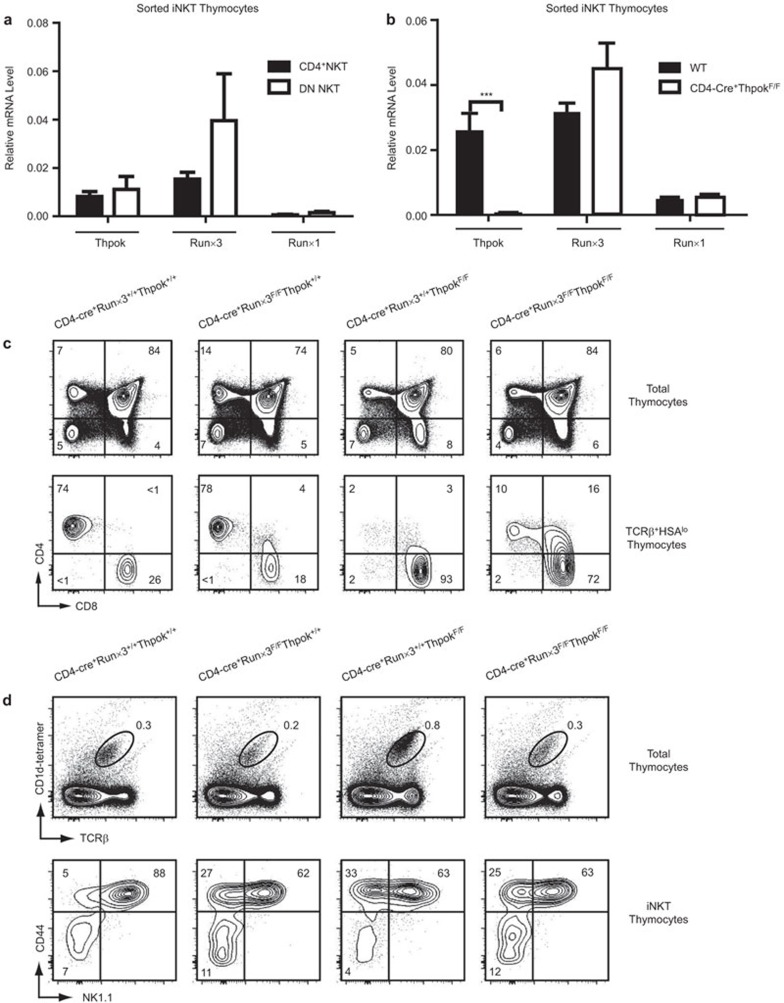
Figure 1.
Altered T-cell phenotype in CD4-cre+Runx3F/F, CD4-cre+ThPokF/F and CD4-cre+Runx3F/FThPokF/F mice. (a) We sorted thymic iNKT cells from WT mice into CD4+ and DN subsets and extracted total RNA to measure the expression levels of ThPok, Runx3 and Runx1 by quantitative real-time PCR. Transcript levels were normalized to the transcript levels of β-actin in each sample. Results are shown as the mean±standard deviation, and data are representative of at least three independent experiments. (b) Determination of mRNA levels of ThPok, Runx3 and Runx1 in sorted thymic iNKT cells from ThPok-deficient and WT mice by flow cytometry and RT-PCR. Results are shown as the mean±standard deviation, and data are representative of three independent experiments. ***P<0.001. (c) Thymocytes from Runx3-deficient, ThPok-deficient, DKO and WT mice were prepared as previously described and analyzed by multicolor flow cytometry. The WT mice are shown as the control; the top row indicates the thymic T-cell phenotype by CD4 versus CD8; in the second row, cells were gated on TCRβ+HSAlow T cells, indicating a population of mature T cells in the thymus. Numbers indicate the percentage of the indicated cell population. Data are representative of more than five independent experiments. (d) Flow cytometric analysis of thymocytes from Runx3-deficient, Thpok-deficient, DKO and age-matched litter mate control mice. The first line shows iNKT cells defined as the αGalCer-CD1d tetramer+TCRβ+ population. Numbers represent the percentage of iNKT cells. The second line depicts the staining of CD1d tetramer+TCRβ+ iNKT cells for NK1.1 and CD44. The latter markers were used to determine the three maturation stages of iNKT cells in the thymus. Data are representative of at least eight independent experiments. αGalCer, α-galactosylceramide; DKO, double knockout; DN, double-negative; iNKT, invariant natural killer T; Runx3, runt-related transcription factor 3; TCR, T-cell receptor; WT, wild-type.