Osteoporosis is a common bone disease that is characterized by decreased bone density and is associated with an increased risk of fractures.1,2 Due to increased life expectancy, the number of patients with osteoporosis, and thus, osteoporosis-associated complications, such as fractures, are expected to increase further in the near future.3 Therefore, the development of new treatment strategies is essential, as treatment with bisphosphonates, which is the gold standard treatment for osteoporosis, is not recommended for and is not well tolerated by all patients.
Recently, Goettsch et al.4 identified nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) as a potential target for the treatment of osteoporosis. The nicotinamide adenine dinucleotide phosphate oxidases represent a family of enzymes that solely produce reactive oxygen species (ROS).5 NOX4 is constitutively active, and therefore, continually produces H2O2. NOX4 is further induced in many cell types during the differentiation process.6 The expression level of NOX4 largely controls the production of ROS.6 ROS have been shown to stimulate osteoclast differentiation and bone resorption in vitro and in vivo.7,8,9,10 Goettsch et al.4 identified NOX4 as the relevant enzymatic source of ROS during bone metabolism and analyzed the mechanisms of osteoclastogenesis induced by ROS.
While osteoblasts mediate bone formation, the osteoclasts promote bone resorption.1 No NOX4-mediated effects on osteoblast function, and thus bone formation, have been observed. However, NOX4 was shown to play an important role in osteoclasts. Bones of Nox4−/− mice were examined and compared to their wild-type (WT) littermates. Nox4−/− mice exhibited a significantly increased trabecular width, thickness and density accompanied by improved biomechanical properties, as determined by the bone strength. These results pointed toward an inhibitory role of NOX4 in bone formation in osteoclasts, as there was no effect on osteoblasts. Thus, Goettsch and colleagues investigated osteoclast markers, such as osteoclast-associated receptor, tartrate-resistant acid phosphatase 5b and carboxyterminal collagen crosslinks. These markers were distinctly reduced in the Nox4−/− mice compared to the WT mice.
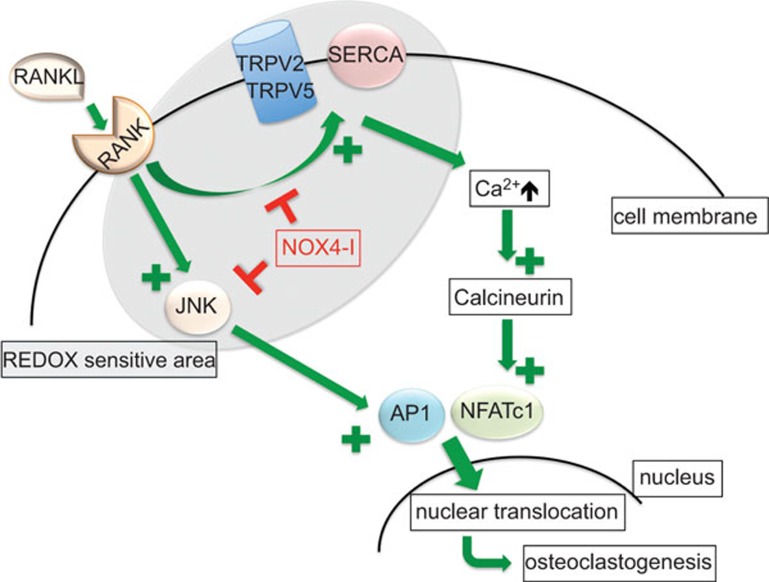
To investigate the effects of NOX4 on osteoclastogenesis, bone marrow mononuclear cells (BMNCs) from Nox4−/− and WT mice were stimulated with macrophage colony-stimulating factor and receptor activator of nuclear factor kappa-B ligand (RANKL), a usual method to induce osteoclastogenesis. In the BMNCs from Nox4−/− mice, a significantly lower number of differentiated osteoclasts were observed. During osteoclastogenesis in the BMNCs from the WT mice, the expression of Nox4 mRNA, and consequently ROS production, increased. In contrast, in the Nox4−/− mice, the ROS production was stable. These results suggest that ROS is produced by NOX4 to mediate the osteoclast differentiation induced by RANKL. Additionally, during macrophage colony-stimulating factor- and RANKL-induced osteoclastogenesis, the cytosolic Ca2+ concentration increased in the WT, but not in the Nox4−/−, BMNCs. This effect on the cytosolic Ca2+ concentration in the WT BMNCs could be prevented by treatment with the NOX4 inhibitor GKT137831. Several transcription factors, such as nuclear factor of activated T cells, cytoplasmic 1 and activator protein 1, are known to be involved in RANKL-induced osteoclastogenesis11 (Figure 1). Both factors were activated in the RANKL-stimulated, macrophage colony-stimulating factor-primed BMNCs from the WT mice, but these factors were not activated in the BMNCs from the Nox4−/− mice, suggesting that NOX4 plays a role their RANKL-induced activation.
Figure 1.
Potential target sites for NOX4 inhibitors within the osteoclastogenesis signaling pathways. The green arrows indicate activation, whereas the effects of the NOX4 inhibitors are indicated in red. AP1, activator protein 1; JNK, c-jun N-terminal kinase; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; NOX4-I, NOX4 inhibitors; RANK, receptor activator of nuclear factor kappa-B; RANKL, receptor activator of nuclear factor kappa-B ligand; REDOX, reduction oxidation; SERCA, sarcoendoplasmatic reticulum Ca2+ ATPase; TRPV2/5, transient receptor potential cation channel V2/5.
Single-nucleotide polymorphism analysis of NOX4 was performed within an existing cohort.12 The rs11018628 single-nucleotide polymorphism, which is located in an intron and could, therefore, affect NOX4 expression, was shown to significantly influence the following bone metabolism markers: alkaline phosphatase, carboxyterminal collagen crosslinks and osteocalcin. All of these markers were increased in subjects carrying the CT or CC alleles when compared to the carriers of the TT allele. Additionally, bone density was analyzed in a subgroup of middle-aged women. Interestingly, a significantly decreased T-score and Z-score of the hip and tendentially decreased hip density were observed in carriers of the CT or CC alleles when compared to carriers of the TT allele.
Furthermore, Goettsch et al. investigated the bones of patients suffering from untreated osteoporosis in comparison with bones from healthy subjects using immunohistochemistry. In the osteoporosis patients, the NOX4 staining intensity was significantly higher, emphasizing the role of NOX4 in this bone disorder. Next, the osteoporosis data were complemented by experiments performed in a mouse model. Female mice were ovariectomized, and as expected, developed osteoporosis after 6 weeks. The Nox4 mRNA and NOX4 protein levels gradually increased in the bones during the bone density loss that occurs after ovariectomy. An acute genetic deletion of Nox4 (tamoxifen-activated Cre recombinase in Nox4fl/fl-ERT-Cre+/0 mice) inhibited the bone loss in the ovariectomized mice. This effect again demonstrated that NOX4 could be a potential target for osteoporosis therapy. To confirm this hypothesis, Goettsch and colleagues treated the ovariectomized WT mice with the NOX4 inhibitor GKT137831. Indeed, the oral, daily administration of the NOX4 inhibitor was able to significantly prevent bone loss.
In summary, NOX4 was induced during the osteoclast differentiation process. The loss of NOX4 activity during osteoclastogenesis halted the differentiation process, and depletion of NOX4 led to increased bone density within the trabecular bone, the typical site of osteoporosis development. Single-nucleotide polymorphism analysis revealed an association of NOX4 expression with bone marker expression. Furthermore, pharmacological inhibition of NOX4 attenuated bone loss in ovariectomized mice. These results suggest that bone loss could be attenuated by the inhibition of NOX4. NOX4 inhibitors13 are currently in preclinical testing and could represent a novel class of future osteoporosis drugs. Although several different anti-osteoporotic agents have been developed such as the gold standard bisphosphonates, there is a need for effective new treatment strategies. Bisphosphonates are not well tolerated in some patients due to gastrointestinal adverse effects; thus, well-tolerated drugs would increase patient compliance. Furthermore, patients suffering from chronic kidney insufficiency, with a glomerular filtration rate below 30 ml/min, cannot be treated with bisphosphonates. Precluding a pharmacological demonstration of a non-kidney-dependent degradation and elimination of NOX4 inhibitors, these drugs could possibly provide a therapeutic option for patients with kidney insufficiency.
References
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68:1218–1225. doi: 10.1093/gerona/glt071. [DOI] [PubMed] [Google Scholar]
- Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68:1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettsch C, Babelova A, Trummer O, Erben RG, Rauner M, Rammelt S, et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest. 2013;123:4731–4738. doi: 10.1172/JCI67603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev. 2013;2013:271602. doi: 10.1155/2013/271602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. . J Clin Invest. 1990;85:632–639. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Putney JW., Jr Calcium signaling in osteoclasts. Biochim Biophys Acta. 2011;1813:979–983. doi: 10.1016/j.bbamcr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, et al. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest. 2009;56:33–41. doi: 10.2152/jmi.56.33. [DOI] [PubMed] [Google Scholar]
- Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Obermayer-Pietsch BM, Bonelli CM, Walter DE, Kuhn RJ, Fahrleitner-Pammer A, Berghold A, et al. Genetic predisposition for adult lactose intolerance and relation to diet, bone density, and bone fractures. J Bone Miner Res. 2004;19:42–47. doi: 10.1359/JBMR.0301207. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]