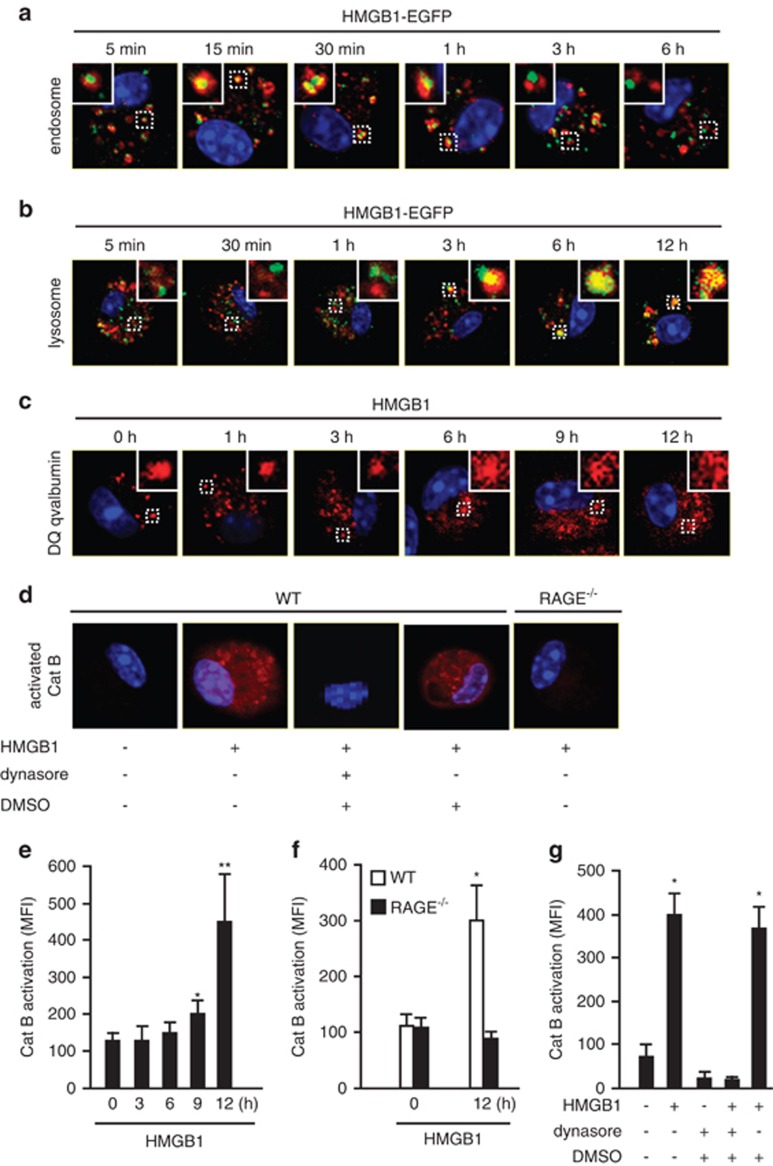
Figure 2.
Localization of HMGB1 in lysosome induces lysosomal destabilization and cathepsin B (CatB) activation. (a) WT AMs were treated with HMGB1-EGFP (green, 100 nmol/l) for 5 min, 15 min, 30 min, 1 h, 3 h, or 6 h, then immunofluorescence of early endosome antigen 1 (red) were detected. Original magnification × 600; results are representative of three independent experiments. (b) AMs were incubated with both HMGB1-EGFP (green, 100 nmol/l) and LysoTracker Red lysosome dye (red) for 5 min, 30 min, 1 h, 3 h, 6 h, or 12 h. Co-localization of HMGB1 and lysosome was detected using confocal microscopy. Original magnification × 600; results are representative of three independent experiments. (c) AMs were treated with HMGB1 (50 nmol/l) for 0–12 h followed by incubation with DQ ovalbumin (red) for 1 h to visualize lysosome integrity using confocal microscopy. Original magnification × 600; results are representative of three independent experiments. (d) AMs isolated from C57BL/6 (WT) or RAGE−/− mice were incubated with HMGB1 (50 nmol/l) for 30 min in the absence or presence of dynasore (30 μg/ml) or DMSO (0.3%). The cells were then stained with Magic Red CatB detection reagent (red) to visualize activated CatB under confocal microscopy. Original magnification × 600; results are representative of three independent experiments. The insets show higher magnification views. (e–g) Macrophages were stained by Magic Red CatB detection reagent to detect the activation of CatB. The mean fluorescence intensity (MFI) of activated CatB was detected and analyzed using confocal microscopy and OLYMPUS Fluoview Ver.1.7c system. (e) WT AMs were stimulated with HMGB1 (50 nmol/l) for 0–12 h (mean and S.D., n=5. *P<0.05 versus the group of 0 h; **P<0.01 versus the group of 0 h). (f) AMs isolated from WT or RAGE−/− mice were stimulated with HMGB1 (50 nmol/l) for 12 h (mean and S.D., n=9. *P<0.05 versus the other groups). (g) WT AMs were stimulated with HMGB1 (50 nmol/l) for 12 h in the absence or presence of dynasore or DMSO (mean and S.D., n=9. *P<0.05 versus the group labeled with no asterisk)