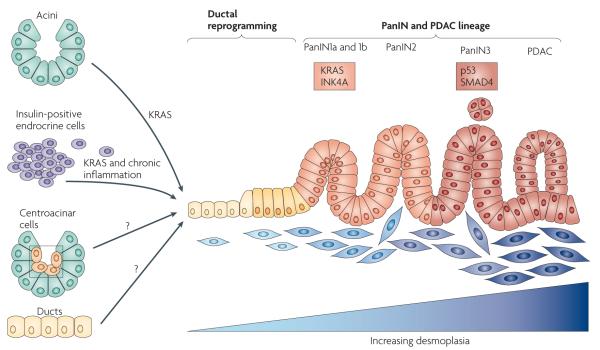
Figure 1. KRAS is a master regulator of pancreatic ductal adenocarcinoma initiation and progression.
Constitutively active KRAS (caused by KrasG12D or KrasG12V mutations) is sufficient to initiate the development of pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC). PanINs are classified into three stages of increasing cellular atypia and, in humans, have been found to possess increasing numbers of mutations (common mutations are indicated in boxes). Changes in the epithelium are matched by desmoplastic changes in the stroma. In mouse models, the human PanIN spectrum followed by progression to PDAC has been recapitulated by activating mutant KRAS in embryonic pancreatic progenitors. Eliminating tumour suppressors commonly inactivated in the human disease dramatically decreases PDAC latency (a limited set of examples is indicated). Mouse models in which KRAS is activated specifically in some adult cell types have shown that both acini and insulin-positive cells can give rise to PanINs and, in some cases, PDAC depending on tissue damage and tumour suppressor inactivation. For these cell types, reprogramming into a ‘ductal’ cell type is required to assume the PanIN–PDAC lineage. Question marks are shown for centroacinar and duct cells as they have not been specifically assessed for their ability to be reprogrammed into a lineage capable of becoming PDAC under the control of KRAS. However, until specific targeting has been achieved, they cannot be ruled out as sources of the precursor–PDAC lineage. Figure is modified, with permission, from REF. 128 © (2000) American Association of Cancer Research.