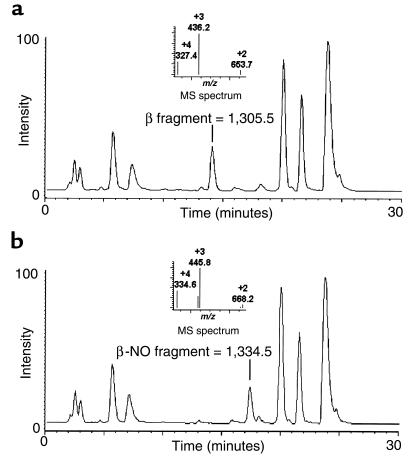
Figure 4.
HPLC-electrospray ionization mass spectrometry of SNO-hemoglobin digested with endoproteinase Glu-C confirms specificity of S-nitrosocysteine reaction with hemoglobin β-chain cysteine 93. (a) Shown is the characteristic chromatogram, after enzymatic digestion of hemoglobin, of the 11–amino acid fragment (hemoglobin β-chain amino acids 91–101) that includes cysteine 93. The molecular weight is 1,305.5, with 3 predominant charge states (+4, +3, +2) with respective m/z of 327.4, 436.2, and 653.7 (inset). (b) Digestion of SNO-hemoglobin results in an increased retention time on the column and an increase in the molecular weight of the 11–amino acid fragment to 1,334.5, consistent with the addition of 29 mass units (NO has 30 mass units minus 1 mass unit of hydrogen lost during covalent binding). The m/z values of the 3 charge states (+4, +3, +2) increase to 334.6, 445.8, and 668.2, respectively (inset).