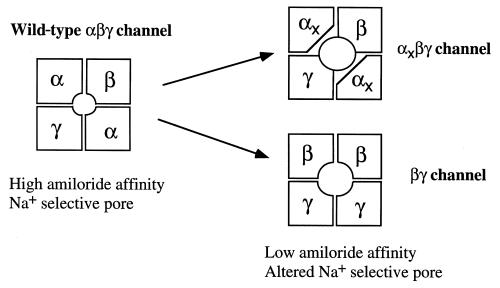
Figure 2.
Possible subunit arrangement of the ion-conducting pore in αxβγ channels and βγ channels. A possible ENaC structure consisting of 2 α, 1 β, and 1 γ subunit is shown as the wild-type channel. A truncated PHA-I mutation, R508x, was created in rat αENaC at the equivalent position (L535x), and αL535x, together with rat β and γ subunits, was expressed in Xenopus oocytes (9). Coexpression of rat β and γ ENaC subunits also generated amiloride-sensitive Na+ currents in oocytes. Interestingly, αL535xβγ and βγ channels displayed very similar macroscopic characteristics, such as reduced amiloride affinity and reduced Li+ selectivity, suggesting that the conductive pores of these channels are composed of P and TM2 domains of β and γ subunits, respectively.