Abstract
The administration of viable Bifidobacterium animalis was tested to induce resistance against Strongyloides venezuelensis infection in mice. Effects on parasite burden, worm length, egg output, and intestinal mucosal histology were evaluated. The oral administration of B. animalis, strain 04450B, starting 14 days before the inoculation of nematode larvae significantly decreased the worm burden and egg output. In probiotic treated animals, the percent reduction of adult worms in the intestine was of 33% and the reduction of egg production was of 21%, compared with those of the control group. The duodenum villous height and villous/crypt ratio were significantly higher in probiotic-treated mice, indicating that this group could be experiencing less intestinal damage. The present findings revealed that the administration of B. animalis for the amelioration of host response to nematode infections is biologically plausible and could have some potential for impacting public health. Meanwhile, further study is needed to delineate the nature and identity of the factor(s) involved in these beneficial effects.
Keywords: Strongyloides venezuelensis, Bifidobacterium animalis, Probiotics, Mice
Abstract
Os efeitos da administração de Bifidobacterium animalis viáveis sobre a infecção por Strongyloides venezuelensis foram avaliados em camundongos experimentalmente infectados. Os parâmetros analisados incluíram a carga parasitária, o comprimento dos vermes, a quantidade de ovos eliminados e a histologia da mucosa intestinal. A administração oral da cepa 04450B de B. animalis, iniciada 14 dias antes da inoculação de larvas do nematódeo, foi acompanhada de uma redução significativa do número de vermes que se estabeleceu no intestino e do número de ovos eliminados nas fezes. Nos animais tratados com o probiótico, o percentual de redução de vermes adultos no intestino foi de 33% e da produção de ovos foi de 21%, em comparação com os do grupo controle. O comprimento das vilosidades do duodeno e a relação vilus/cripta foram significativamente maiores nos animais tratados, indicando que nestes animais as lesões intestinais foram mais leves. Os resultados do presente trabalho revelaram que a administração de B. animalis com o propósito de modular a resposta do hospedeiro contra infecções por nematódeos é uma possibilidade biologicamente plausível com impacto potencial em saúde pública. No entanto, são ainda necessários mais estudos para esclarecer os mecanismos de ação destes microrganismos e identificar os fatores envolvidos na produção dos efeitos benéficos.
INTRODUCTION
In recent years, there have been remarkable advances in the understanding of the ecology, epidemiology, and morbidity related to helminthic infections, which have pointed to the need for new intervention tools, focusing especially on three main aspects: the improvement of the nutritional status of the host, the prevention or treatment of infection, and the improvement of immunocompetence20. Indeed, there has been a significant upsurge in research on the characterization of probiotic bacteria and the evaluation of the potential health benefits associated with the use of these microorganisms as functional food.
Probiotics are defined as live microbial food supplement microorganisms that confer health benefits when administered in adequate amounts15. In this way, the overall focus of probiotic research is to assess if dietary microrganisms can safely enhance immune function and gut health by improving the immune response against infectious agents.
The gastrointestinal helminth Strongyloides stercoralis currently infects an estimated 30-100 million people worldwide. This infection ranges from an asymptomatic clinical presentation to a severe lifethreatening condition in certain population subgroups such as patients under immunosuppressive therapy and individuals with HIV/AIDS. In developing countries, where malnutrition is one of the most important causes of immunodeficiency, mainly among children, this clinical condition has been found to be associated with severe strongyloidiasis27.
Humans are the natural host of S. stercoralis, and up to now, the attempts made to develop an appropriated animal model have been unsuccessful, since this parasite cannot complete its life cycle in immunologically intact mouse strains10. Otherwise, Strongyloides venezuelensis, a nematode isolated from wild rats, is considered a suitable model of strongyloidiasis, as following the infection, its larvae migrate through the lungs before establishing themselves in the duodenal mucosa, evoking innate and acquired immune responses similar to those observed in human infection by S. stercoralis. In human hosts and in murine models, the immune response to Strongyloides spp. is characterized by intraepithelial and tissue increase of eosinophils, as well as by intestinal mastocytosis and production of Th2-type cytokines31. Moreover, S. venezuelensis is safe to be used in experimental assays, being a useful biological model to investigate several aspects of host–parasite relationship, including the efficacy of therapeutic agents24.
Lately, several probiotic microorganisms have been evaluated as single agents or combination therapies in a wide range of infectious and non-infectious diseases. Studies on probiotics have revealed that these microorganisms contribute to host defense by reinforcing non-immunological responses and stimulating both specific and non-specific host immunity14. Probiotic bacteria have successfully been tested in intestinal protozoan and helmintic infections such as cryptosporidiosis29, giardiasis6, coccidiosis9, trichinellosis3 and toxocariasis3,7.
The main organisms employed as probiotics are lactic acid bacteria, especially of the genus Lactobacillus and Bifidobacterium. They occur naturally as part of the normal gut flora, having a long history of safe use in food and fermented products14,30. Taking into account this particular aspect and all that has been mentioned above, Bifidobacterium animalis, one of the main well-characterized probiotic strains that is commercially available, was used in the present study to assess their positive effects on S. venezuelensis infection. The S. venezuelensis mouse model was chosen, considering that the infection leads to a local immune response at intestine mucosal surface, and an allergic response in the lung, both proposed to be modulated by probiotic administration.
MATERIALS AND METHODS
Animals
Four-week-old male BALB/c inbred mice were purchased from the Centro Multidisciplinar para Investigação Biológica na Área da Ciência em Animais de Laboratório (CEMIB), Universidade Estadual de Campinas, SP, Brazil. Twenty-eight animals were housed in plastic cages, fed a standard diet, and allowed free access to water. The experimental procedures were approved by the Animal Ethics Committee of College of Veterinary Medicine and Animal Science (UNESP).
Bacteria: Bifidobacterium animalis strain 04450B was kindly provided by Danisco Brasil Ltda. The probiotic was grown on De Man-Rogosa-Sharpe broth (Oxoid) adding NA2CO3 (0.02%), CaCl2.2H2O (0.01%), and L-cysteine hydrochloride (0.05%) under anaerobic conditions at 37° C for 16 hours. Following culture, the medium was centrifuged at 3000g, and the pelleted organisms were resuspended in 10% sterile milk to give a final concentration of 2 x 109 CFU/mL.
Nematode: The strain of Strongyloides venezuelensis was isolated from a wild rat in the early ‘80s in Botucatu, São Paulo State, and has been maintained in the laboratory by repeated passages in Wistar rats. Filariform larvae (L3) were obtained from fecal cultures and adjusted to the appropriate number in distilled water in order to infect the animals23.
Experimental procedure: The animals were randomly allocated in groups of seven mice, distributed as follows: two probiotic treated groups (PB1 and PB2) and two non-treated control groups (C1 and C2). Daily, the animals of the groups PB1 and PB2 received one mL of milk containing 2 x 109 UFC of B. animalis by gavage. The control groups of mice (C1 and C2) received the same volume (one mL) of skim milk. These procedures were repeated until the animals had been killed.
Fourteen days after the beginning of the experiment, 2,000 infective larvae (L3) of S. venezuelensis were subcutaneously inoculated in all animals of the four groups, according to AMARANTE & OLIVEIRA-SEQUEIRA1. For collection of fecal samples, the animals of probiotic treated (PB1 and PB2) and control (C1 and C2) groups were allocated in boxes where they stayed for three hours. The feces obtained from each group constituted a sample for fecal egg counts (FEC). Fecal egg counts were daily assessed by the modified McMaster technique16 in which, each egg counted represents 100 eggs per gram of feces (EPG).
Six days after the inoculation of the nematode infective larvae, the mice of PB1 and C1 groups were killed. The small intestine (SI) was removed and a segment of one cm was sectioned (2-6 cm from the pyloric ring) and immersed in Boin's fixative for histological analysis. For the recovery and counting of adult worms, the remaining small intestine was inverted over a thin wire support, placed in 20 mL tubes containing phosphate-buffered saline (PBS), and incubated for four hours at 37 °C1. The wire supports with intestines were then vigorously shaken and removed from tubes. Supernatants were discharged and the sediment containing the worms was preserved in formaldehyde 5%. The nematodes were counted under stereoscopic microscopy. To determine the worms' length, 20 partenogenetic females recovered from each animal were measured using a computerized image analysis system (QWin Lite 2.5, Leica), adapted in DMLB light photomicroscope (Leica).
The fecal egg counts were carried out for the remaining animals of groups PB2 and C2 until the 14th day, when they were killed. At the necropsy, the small intestine was removed and processed as previously described.
Histological analysis: The fixed fragments of duodenum tissues were embedded in paraffin, and cut into 3-5 µm thick sections. The sections stained with hematoxylin-eosin were used for histopathological analysis and for villous height and crypt depth measurements. Villous height was measured from the tip to the crypt junction, and the crypt depth was defined as the depth of the invagination between adjacent villi. The ratio of villous/crypt length (v/c) was also calculated. The villous and crypt lengths measurements were performed in 10-crypt units of each sample, as recommended by IERNA et al. 19. These results were expressed as a mean of each group.
Statistical analysis: The two-tailed Student's t-test was employed to evaluated group differences in relation to parasitological parameters (worm burden and female length), and the differences related to histological parameters (crypt and villous length) were analyzed by Mann-Whitney U test. p values < 0.05 were considered statistically significant.
RESULTS
Parasitological parameters
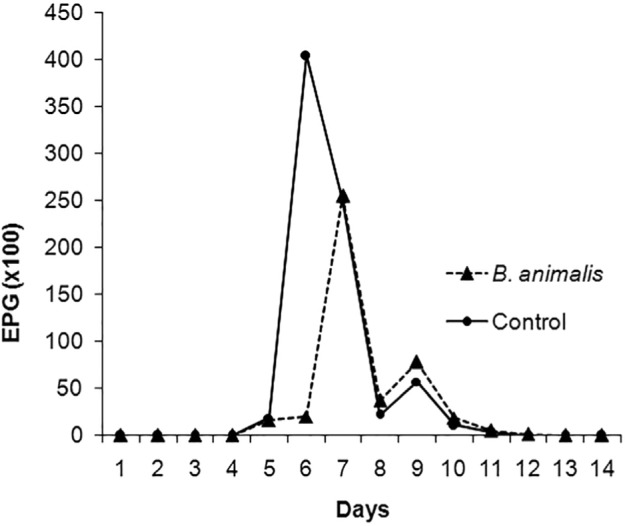
Kinetics of fecal egg counts (FEC) in S. venezuelensis infected mice (probiotic-treated and control groups) are shown in Fig. 1. In both animal groups, eggs were first detected in feces five days after larval subcutaneous inoculation. In probiotic-treated animals, egg output peaked on day 7 when it reached 25,500 EPG. In the control group, the peak occurred on day 6, reaching 40,400 EPG. The patent period of infection was seven and eight days, respectively, for control and probiotic-treated groups. The total FEC at the end of the patent period was 60,367 and 76,433 EPG in probiotic treated and control groups, respectively.
Fig. 1. Kinetics of FEC in S. venezuelensis infected mice probiotic-treated and control groups. 177x133 mm (100 x 100 DPI).
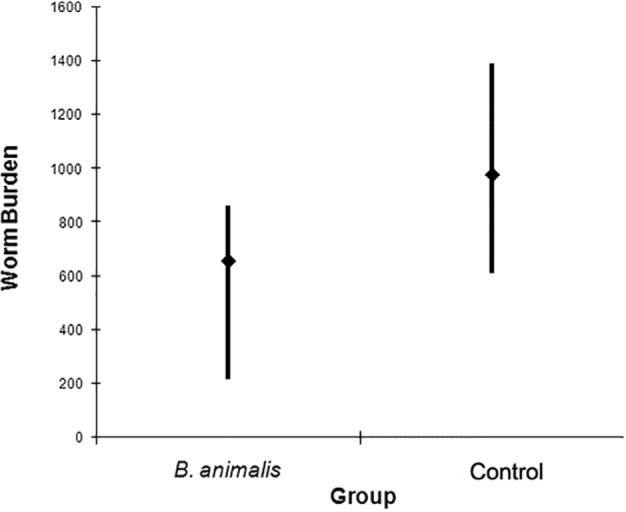
On day 6 after infection, the worm burden assessment (Fig. 2) revealed that the number of partenogenetic females (5,235) recovered from all seven animals of B. animalis-treated group was lower than that from the control one (7,819). The mean number of females recovered from probiotic-treated mice was 654 (± 137) while in the control group it was 977 (± 183). In B. animalis-treated mice the minimum (216) and maximum (608) numbers of parasitic females recovered were lower than those from control animals, which ranged from 608 to 1,388 worms. All these differences were statistically significant (p < 0.05).
Fig. 2. Mean, minimum and maximum numbers of S. venezuelensis partenogenetic females recovered from probiotic-treated and control groups of mice. 163x133 mm (100 x 100 DPI).
The female length recovered from probiotic-treated mice ranged from 1,860 to 4,082 µm (2,943 ± 128 µm), whereas those from control mice ranged from 2,319 to 3,781 µm (3,025 ± 151 µm), showing no statistical differences (p = 0.25).
Histological analysis
In the sections of duodenum collected at the 6th day after infection (dpi), mature worms were found in the lumina of the gut, lodged in spaces between mucosal epithelial cells and into the lamina propria (LP) of the villi. At this time, in mice of both PB1 and C1 groups, the presence of worms was accompanied by a diffuse cellular infiltration consisting of polimorphonuclear eosinophils and mononuclear cells. These inflammatory cells were sparsely distributed in the basal zone of LP, while at tip of the villi, these cells were clustered. Blood vessels at the tip of the villi showed some degree of congestion together with interstitial edema. Besides, the epithelial cells of the top of the villi showed a slight level of vacuolar degeneration. In the mice killed at 14th dpi, no worms were seen in the intestine, either in probiotic-treated or in control mice, but the same kind of cellular infiltration observed at 6th dpi was still present.
The villous height, crypt depth, and villous/crypt ratio in the upper small intestine of control and probiotic-treated mice on the 6th (PB1 and C1) and 14th (PB2 and C2) days after infection with S. venezuelensis are shown in Table 1. Probiotic administration did not affect crypt depth, but the associated villous height and villous/crypt ratio were significantly greater in probiotic-treated mice killed at 6th dpi (PB1) when compared to its respective control group (C1) and to probiotic-treated (PB2) and control (C2) groups, killed at 14th dpi.
Table 1. Effect of probiotic treatment on villous height and crypt depth (µm), and villous/crypt ratio in mice infected with S. venezuelensis .
| Groups | Villous height (Mean ± SEM) | Crypt depth (Mean ± SEM) | Villous/crypt ratio |
|---|---|---|---|
| PB1 | 477.1(86.4)a | 236.8(33.2) | 2.01a |
| PB2 | 358.7(42.7)b | 254.6(58.0) | 1.41b |
| C1 | 387.9(69.3)b | 264.3(71.4) | 1.47b |
| C2 | 374.1(57.5)b | 279.0(60.5) | 1.34b |
Values followed by different letters in columns are significantly different (p < 0.05)
DISCUSSION
Helminth infections are among the most common infections in humans. In developing countries, this infection is frequently associated with malnutrition, representing a significant cause of child developmental retardation. On the other hand, in most developing countries, there are public school feeding programs designed to avoid malnutrition focusing on complementary nutrition, child intestinal parasite control or both35. Therefore, the present study was an attempt to investigate the effect of probiotic supplementation on the modulation of helminth infection using a murine model.
Several clinical studies have demonstrated the therapeutic and/or prophylactic efficacy of specific probiotics against acute viral gastroenteritis and antibiotic-associated diarrhea. The studies on the protection afforded probiotics have been performed mainly with bacterial enteropathogens, but it has been suggested that they may also influence the degree of parasitic infection such as those due to Giardia 5,18, Eimeria 9,22 and Cryptosporidium 29.
In the present study, the administration of B. animalis to S. venezuelensis infected mice triggered a protective effect, characterized by a reduced number of worms and also by a reduced egg output. The percentage of adult worm reduction in the intestine, six days after S. venezuelensis infection, was 33% of mice treated with B. animalis. In the same way, the reduction of egg production in the probiotic-treated mice was 21%. Interestingly, the reduction of egg output was especially significant in the peak of elimination, when besides the reduction of 37%, peak delayed one day in probiotic treated mice in relation to control mice. Female length and pre-patent period were not affected by the probiotic administration, but the patent period was one day longer in probiotic-treated mice.
In relation to probiotic effects on helminth infections, the few available studies provide conflicting results. Reduction of worm burden was reported in mice infected with Trichinella spiralis 3,4,25 and Toxocara canis 2,7. The failure of probiotics in affecting parasite load was reported in rats12 and mice33 infected with T. spiralis, and finally, an enhancement of mice susceptibility to Trichuris muris was attributed to Lactobacillus casei administration11.
The overall evaluation of the effect of probiotic administration on the worm burden revealed that is difficult to compare results, because of differences in study design, animal model, probiotic dose and strain, and administration route. Nevertheless, most of the available studies revealed promising results, encouraging further investigations in order to confirm the real role of probiotics in host response to nematode infections.
Worm burden is the most frequently investigated aspect in terms of host response to helminth infections, but this response may also interfere with some of the physiological functions of the parasite, including reduction of parasite fertility, limited growth and structural damage21. Our data revealed no effect of probiotic administration on the growth of female recovery on the 6th day after infection (p = 0.26). So, instead of being related to poor female growth, the observed reduction of egg output may be related to worm burden, because fecal egg counts are correlate highly with parasite load26. This is an important observation because egg production is the main means by which the parasite is known to spread. Reduction of fecal shed forms like cysts and oocysts associated with probiotic administration was previously reported in Giardia 32 and Eimeria acervulina infections8,9,22.
The beneficial claims of probiotics activities are poorly understood, needing scientific validation that can be addressed by assessing probiotic strains under controlled experimental conditions. In order to understand the immunomodulatory mechanism by which probiotic administration improves the host response against S. venezuelensis infection, some components of the intestinal response considered relevant against nematode infection were investigated.
In the present work, eosinophils and mononuclear were the main cells infiltrating the intestinal mucosa of mice, showing similar patterns of distribution in probiotic-treated mice and control group, either after six or fourteen days after the inoculation of the S. venezuelensis larvae. Increased eosinophils and mononuclear cell numbers are commonly associated with helminth infections, and in S. stercoralis infection of mice, their role as antigen-present cell for the induction of the primary and expansion of secondary Th2 immune response has been characterized28. According to the data obtained here, B. animalis administration appears to make no difference in patterns of effector cells infiltration and spatial distribution. Therefore, the effect of the probiotic administration in reducing the worm burden and the egg output in B. animalis treated mice cannot be attributed to a modulation of these inflammatory cells, at least at the intestinal level. It would be of great interest to know whether B. animalis has an effect upon these cells during lung migrating stages, since S. venezuelensis infection also increases eosinophil and mononuclear cell numbers in blood and bronchoalveolar fluid23. Besides, it appears that activated eosinophils are relevant for the destruction of migrating larvae, rather than for the elimination of adult worms13.
Villous atrophy and/or crypt hyperplasia are occasionally induced by nematode infection17 and high apoptotic rates concomitant with low cell proliferation was found associated with a S. stercoralis infection in humans34. On the other hand, it has been demonstrated that the administration of either Bifidobacterium adolescentis or Bifidobacterium longum increase the height of duodenum villi36. Thus, in the present work, the measurement of villous height and crypt depth was performed in order to verify if probiotic administration has some effect in repairing the intestinal epithelium during S. venezuelensis infection.
Data obtained here revealed that the duodenum villous height and villous/crypt ratio were significantly higher in probiotic-treated mice killed six days after S. venezuelensis infection, indicating that this group could be experiencing less intestinal damage. Fourteen days after infection, when most of the worms were expelled, no difference in villous length or villous/crypt ratio was found in probiotic-treated and control mice. A time declining effect of the administration of Bifidobacterium longum upon the mice's villous length was previously reported36.
Taken together, the present findings suggest that the administration of B. animalis to improve immunity to nematode infections is biologically plausible and could have some impact in public health. Considering that intestinal parasitism and malnutrition share a similar geographical distribution, studies with probiotics should focus on the development of functional foods for the treatment of both diseases.
REFERENCES
- 1.Amarante AFT, Oliveira-Sequeira TCG. Strongyloides venezuelensis infection susceptibility of seven inbred strains of mice. Arq Bras Med Vet Zootec. 2002;54:273–8. [Google Scholar]
- 2.Basualdo J, Sparo M, Chiodo P, Ciarmela M, Minvielle M. Oral treatment with a potential probiotic (Enterococcus faecalis CECT 7121) appears to reduce the parasite burden of mice infected with Toxocara canis . Ann Trop Med Parasitol. 2007;101:559–62. doi: 10.1179/136485907X193824. [DOI] [PubMed] [Google Scholar]
- 3.Bautista-Garfias CR, Ixta-Rodríguez O, Martínez-Gómez F, López MG, Aguilar-Figueroa BR. Effect of viable or dead Lactobacillus casei organisms administered orally to mice on resistance against Trichinella spiralis infection. Parasite. 2001;8((2 Suppl)):S226–8. doi: 10.1051/parasite/200108s2226. [DOI] [PubMed] [Google Scholar]
- 4.Bautista-Garfias CR, Ixta O, Orduña M, Martínez F, Aguilar B, Cortés A. Enhancement of resistance in mice treated with Lactobacillus casei: effect on Trichinella spiralis infection. Vet Parasitol. 1999;80:251–60. doi: 10.1016/s0304-4017(98)00210-6. [DOI] [PubMed] [Google Scholar]
- 5.Benyacoub J, Pérez PF, Rochat F, Saudan KY, Reuteler G, Antille N, et al. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr. 2005;135:1171–6. doi: 10.1093/jn/135.5.1171. [DOI] [PubMed] [Google Scholar]
- 6.Besirbellioglu BA, Ulcay A, Can M, Erdem H, Tanyuksel M, Avci IY, et al. Saccharomyces boulardii and infection due to Giardia lamblia . Scand J Infect Dis. 2006;38:479–81. doi: 10.1080/00365540600561769. [DOI] [PubMed] [Google Scholar]
- 7.Chiodo PG, Sparo MD, Pezzani BC, Minvielle MC, Basualdo JA. In vitro and in vivo effects of Enterococcus faecalis CECT7121 on Toxocara canis . Mem Inst Oswaldo Cruz. 2010;105:615–20. doi: 10.1590/s0074-02762010000500003. [DOI] [PubMed] [Google Scholar]
- 8.Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. 2005;28:351–61. doi: 10.1016/j.cimid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult Sci. 2003;82:62–6. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- 10.Dawkins HJ, Grove DI. Attempts to establish infections with Strongyloides stercoralis in mice and laboratory animals. J Helminthol. 1982;56:23–6. doi: 10.1017/s0022149x00034957. [DOI] [PubMed] [Google Scholar]
- 11.Dea-Ayuela MA, Rama-Iñiguez S, Bolás-Fernandez F. Enhanced susceptibility to Trichuris muris infection of B10Br mice treated with the probiotic Lactobacillus casei . Int Immunopharmacol. 2008;8:28–35. doi: 10.1016/j.intimp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.de Waard R, Garssen J, Snel J, Bokken GC, Sako T, Veld JH, et al. Enhanced antigen-specific delayed-type hypersensitivity and immunoglobulin G2b responses after oral administration of viable Lactobacillus casei YIT9029 in Wistar and Brown Norway rats. Clin and Diagn Lab Immunol. 2001;8:762–7. doi: 10.1128/CDLI.8.4.762-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Malky M, Maruyama H, Hirabayashi Y, Shimada S, Yoshida A, Amano T, et al. Intraepithelial infiltration of eosinophils and their contribution to the elimination of adult intestinal nematode Strongyloides venezuelensis in mice. Parasitol Int. 2003;52:71–9. doi: 10.1016/s1383-5769(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 14.Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2003;17:755–73. doi: 10.1016/s1521-6918(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 15.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–8. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- 16.Gordon HM, Whitlock HV. A new technique for counting nematode eggs in sheep faeces. J Counc Sci Ind Res. 1939;12:50–2. [Google Scholar]
- 17.Hashimoto K, Uchikawa R, Tegoshi T, Takeda K, Yamada M, Arizono N. Depleted intestinal goblet cells and severe pathological changes in SCID mice infected with Heligmosomoides polygyrus . Parasite Immunol. 2009;31:457–65. doi: 10.1111/j.1365-3024.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 18.Humen MA, De Antoni GL, Benyacoub J, Costas ME, Cardozo MI, Kozubsky L, et al. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo . Infect Immun. 2005;73:1265–9. doi: 10.1128/IAI.73.2.1265-1269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ierna MX, Scales HE, Mueller C, Lawrence CE. Transmembrane tumor necrosis factor alpha is required for enteropathy and is sufficient to promote parasite expulsion in gastrointestinal helminth infection. Infect Immun. 2009;77:3879–85. doi: 10.1128/IAI.01461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu Rev Nutr. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 21.Krupp IM. Effects of crowding and superinfection on habitat selections and egg production in Ancylostoma caninum . J Parasitol. 1961;47:957–61. [PubMed] [Google Scholar]
- 22.Lee SH, Lillehoj HS, Dalloul RA, Park DW, Hong YH, Lin JJ. Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poult Sci. 2007;86:63–6. doi: 10.1093/ps/86.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Machado ER, Carlos D, Lourenço EV, Sorgi CA, Silva EV, Ramos SG, et al. Counterregulation of Th2 immunity by interleukin 12 reduces host defenses against Strongyloides venezuelensis infection. Microbes Infect. 2009;11:571–8. doi: 10.1016/j.micinf.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Machado ER, Ueta MT, Gonçalves-Pires MR, de Oliveira JB, Faccioli LH, Costa-Cruz JM. Diagnosis of human strongyloidiasis using particulate antigen of two strains of Strongyloides venezuelensis in indirect immunofluorescence antibody test. Exp Parasitol. 2001;99:52–5. doi: 10.1006/expr.2001.4632. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Gómez F, Santiago-Rosales R, Bautista-Garfias CR. Effect of Lactobacillus casei Shirota strain intraperitoneal administration in CD1 mice on the establishment of Trichinella spiralis adult worms and on IgA anti-T. spiralis production. Vet Parasitol. 2009;162:171–5. doi: 10.1016/j.vetpar.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira-Sequeira TCG, Amarante AFT. Dynamics of Strongyloides venezuelensis infection and relationship between fecal egg counts and parasite burden in Swiss mice. Rev Bras Med Vet. 2001;23:99–102. [Google Scholar]
- 27.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis: the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967–72. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, et al. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis. 2007;196:1844–51. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickerd N, Tuthill D. Resolution of cryptosporidiosis with probiotic treatment. Postgrad Med J. 2004;80:112–3. doi: 10.1136/pmj.2003.014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues RM, Silva NM, Gonçalves AL, Cardoso CR, Alves R, Gonçalves FA, et al. Major histocompatibility complex (MHC) class II but not MHC class I molecules are required for efficient control of Strongyloides venezuelensis infection in mice. Immunology. 2009;128:432–41. doi: 10.1111/j.1365-2567.2008.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla G, Devi P, Sehgal R. Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci. 2008;53:2671–9. doi: 10.1007/s10620-007-0197-3. [DOI] [PubMed] [Google Scholar]
- 33.Verdú EF, Bercík P, Bergonzelli GE, Huang XX, Blennerhasset P, Rochat F, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127:826–37. doi: 10.1053/j.gastro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Werneck-Silva AL, Alvares EP, Gama P, Damião AO, Osaki LH, Ogias D, et al. Intestinal damage in strongyloidiasis: the imbalance between cell death and proliferation. Dig Dis Sci. 2006;51:1063–9. doi: 10.1007/s10620-006-8010-2. [DOI] [PubMed] [Google Scholar]
- 35.Worku N, Erko B, Torben W, Belay M, Kasssu A, Fetene T, et al. Malnutrition and intestinal parasitic infections in school children of Gondar, North West Ethiopia. Ethiop Med J. 2009;47:9–16. [PubMed] [Google Scholar]
- 36.Yang H, Liu A, Zhang M, Ibrahim SA, Pang Z, Leng X, et al. Oral administration of live Bifidobacterium substrains isolated from centenarians enhances intestinal function in mice. Curr Microbiol. 2009;59:439–45. doi: 10.1007/s00284-009-9457-0. [DOI] [PubMed] [Google Scholar]


