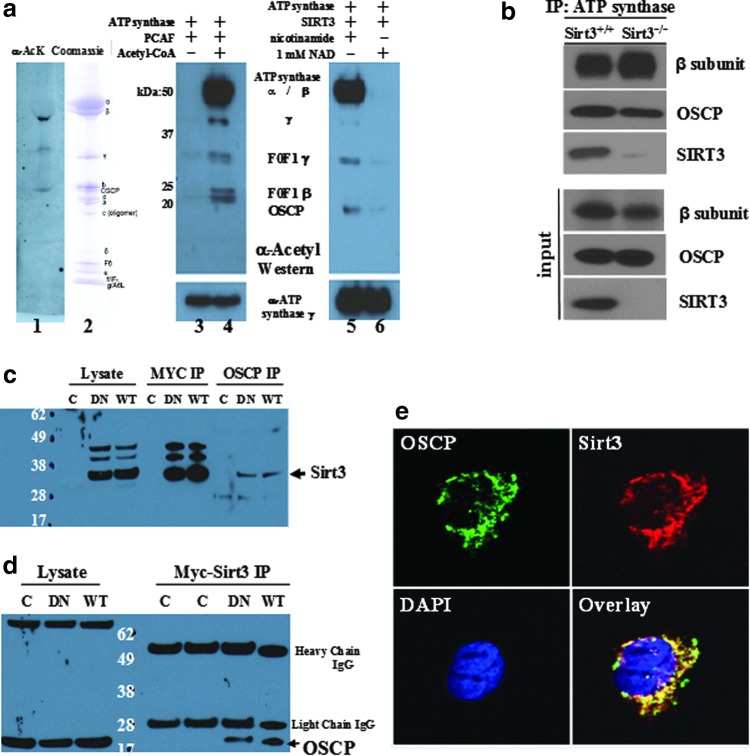
FIG. 1.
The adenosine diphosphate (ATP) synthase complex contains multiple proteins with reversible acetyl-lysines and interacts with SIRT3. (a) Left panel, two 50 μg samples of highly purified bovine F1Fo ATP synthase were separated, transferred, and blotted with an acetyl-lysine antibody (Cell Signaling, Inc.) or stained with Coomassie. Middle panel, purified bovine F1Fo ATP synthase was treated with PCAF and blotted. Right panel, purified bovine F1Fo ATP synthase treated with PCAF was purified, treated with recombinant human SIRT3, and blotted with an acetyl-lysine antibody. (b) Skeletal muscle mitochondrial lysates from Sirt3+/+ and Sirt3−/− mice were harvested, IPed with an anti-ATP synthase antibody (Abcam, Inc.), and subsequently immunoblotted with an anti-OSCP antibody (Santa Cruz Biotechnology, Inc.), anti-ATP synthase β subunit (Abcam, Inc.), or anti-SIRT3 antibody (Cell Signaling, Inc.). (c-d) SIRT3 physically interacts with oligomycin sensitivity-conferring protein (OSCP). HCT116 cells were constructed to constitutively express a Myc-tagged SIRT3 gene, and cell extracts were (c) IPed with either an anti-Myc or an OCSP antibody and immunoblotted with an anti-SIRT3 antibody, or (d) IPed with an anti-Myc antibody and immunoblotted with an OCSP antibody. (e) IHC staining with an anti-SIRT3 and anti-OSCP antibody, with the images merged in the lower right section. Representative micrographs are shown.