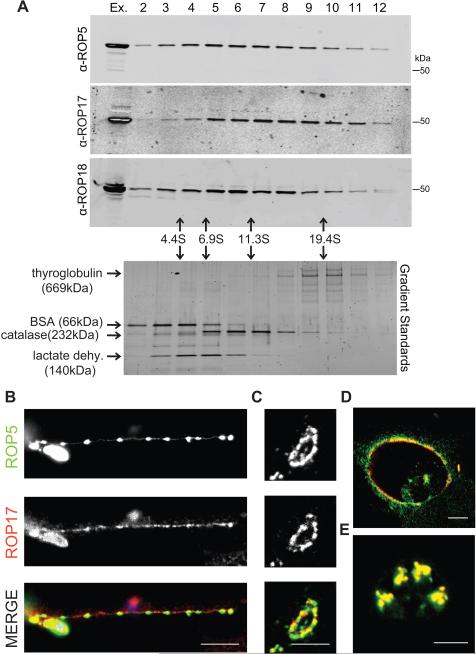
Figure 2. Characterization of the rhoptry kinase complexes.
(A) Glycerol gradient separation of ROP kinases. Whole cell extracts of T. gondii were fractionated on 10-30% glycerol gradients, resolved on 8-16% SDS-PAGE gels, Western blotted with antibodies shown, and detected using LI-COR specific secondary antibodies. Sedimentation coefficients were determined using standards run in parallel, lower gel stained with SYPRO Ruby. (B) Localization of ROP17 in infected host cells. RHΔku80 T. gondii parasites were used to challenge HFF monolayers for 30 min in 1μM cytochalasin D to block invasion and visualize proteins secreted into evacuoles. Monolayers were fixed and permeabilized with 0.05% saponin. Alternatively, parasites were allowed to invade HFF cells without cytochalasin D for 30 min (C) or 16 hr (D), fixed, and permeabilized with 0.002% digitonin to selectively expose the cytosolic surface of the PVM. (E) To detect proteins localized to the rhoptries, HFF monolayers were fixed at 16 hr post infection and fully permeabilized with 0.1% TritonX-100. Samples were incubated with mouse anti-ROP17, visualized by goat anti-mouse IgG conjugated to Alexa 594 (red), and rabbit antisera to ROP5, visualized by goat anti-rabbit IgG conjugated to Alexa 488 (green). Scale bars = 5 microns. Representative of two similar experiments. See also Figure S1.