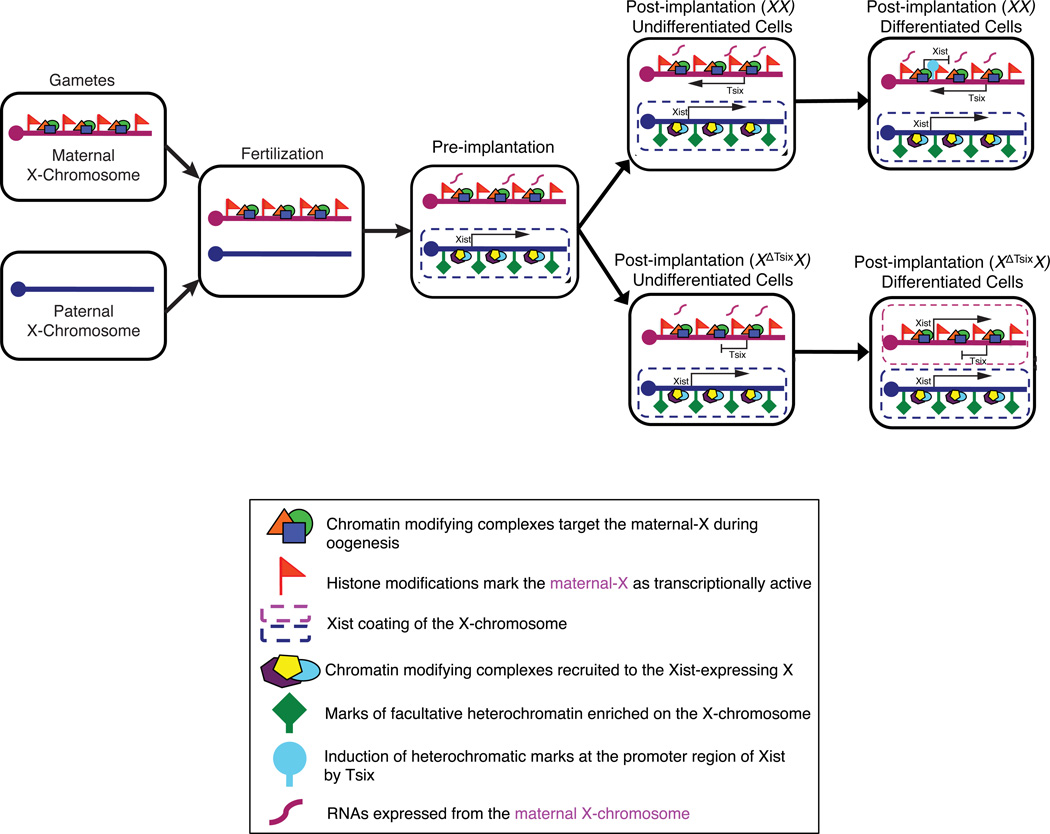
Figure 8. A model for the role of Tsix in imprinted X-Inactivation.
The maternal X-chromosome, but not the paternal-X, is marked by histone modifications during gametogenesis that are transmitted to the offspring upon fertilization. In the pre-implantation embryo, these histone modifications prevent inactivation of the maternal-X, while the paternal X-chromosome is subject to inactivation. Xist is induced from the paternally-derived X-chromosome in the pre-implantation embryo, and helps recruit protein complexes that catalyze histone marks characteristic of facultative heterochromatin on the paternal-X. The oocyte-configured chromatin of the maternal-X, conversely, prevents Xist induction from the maternal X-chromosome during the initiation phase of X-inactivation (pre-implantation) and does not require Tsix. The maternal-X then remains active during the maintenance phase of imprinted X-inactivation in undifferentiated extra-embryonic nuclei (post-implantation, undifferentiated cells), independently of Tsix expression. Tsix is induced from the maternal-X due to the absence of Xist expression from this X-chromosome. Upon differentiation, Tsix transcription across the Xist promoter region is required to induce heterochromatinization of the Xist promoter to keep Xist silenced in the extra-embryonic trophectodermal lineage. In XX differentiated extra-embryonic cells, the wild-type maternal-X remains transcriptionally competent while the paternal-X is maintained as transcriptionally inactive in imprinted X-inactivated cells. Upon differentiation of XΔTsixX trophectoderm cells, the Tsix-mutant maternal X-chromosome induces Xist.