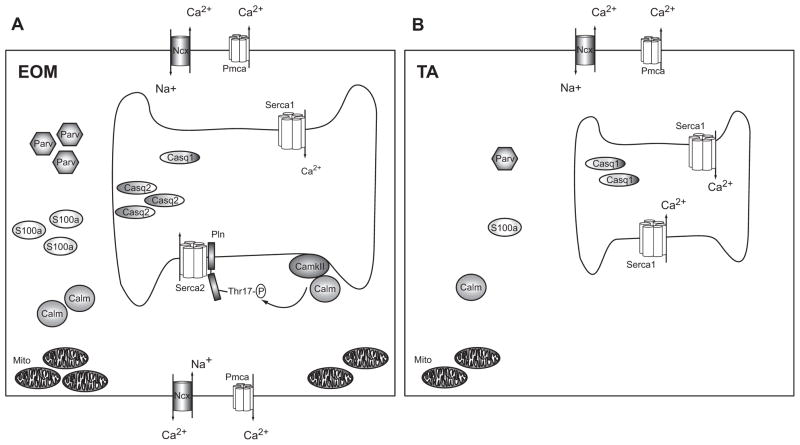
Fig. 6. Model for superior Ca2+ homeostasis in EOM.
Schematic (A) shows an EOM muscle cell with a larger SR, numerous pumps and transporters in both the sarcolemma and the sarcoplasmic membrane and many Ca2+ binding proteins in the cytosol (e.g. S100a, Parv) and SR (Casq1 and Casq2), as well as numerous mitochondria. CamkII phosphorylates Pln at Thr17, which regulates Serca2. Schematic (B) represents a TA muscle cell containing a smaller SR, fewer pumps and transporters and less Ca2+ binding proteins in the cytosol and SR compared with EOM. Excess Ca2+ ions entering the cell can be bound and/or removed more effectively in EOM than TA, leading to a faster return to resting Ca2+ levels. We propose that the extensive Ca2+ handling capacity of EOM reflects their functional properties and may help to protect this muscle group from Ca2+ mediated damage in DMD. (Symbols: Serca: sarcoplasmic reticulum Ca2+-ATPase, Casq: Calsequestrin, Pln: Phospholamban, Pmca: plasmamembrane Ca2+-ATPase, Parv: Parvalbumin, CamkII: Ca2+/Calmodulin dependent kinase II, Calm: Calmodulin, Mito: Mitochondria, Ncx: Na+/Ca2+-exchanger, Thr17-P: phosphorylated threonine 17 residue).