Table 2.
SAR study of the A-ring of 1,2,4-bis-aryloxadiazole. Expression of an AHR response gene, Cyp1a1, was measured in HC11 MECs treated with 10 μM compound for 48 hours.
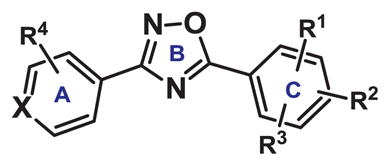
| ||||||
|---|---|---|---|---|---|---|
| Compound | X | R1 | R2 | R3 | R4 | Relative Cyp1a1 |
| 11 | CH | o-Cl | p-Cl | H | m-CF3 | 1.84 +/− 0.28 |
| 12 | CH | o-Cl | p-Cl | H | m-CO2Me | 0.18 +/− 0.49 |
| 13 | CH | o-F | H | H | m-CO2Me | 0.29 +/− 0.74 |
| 14 | CH | o-F | H | H | m-CO2H | 3.71 +/− 0.22 |
| 15 | CH | o-Cl | p-Cl | H | o-Cl | 5.91 +/− 0.18 |
| 16 | CH | o-Cl | p-Cl | H | m-Cl | 1.59 +/− 0.30 |
| 17 | CH | o-Cl | p-Cl | H | p-Cl | 8.15 +/− 0.14 |
| 18 | CH | o-Cl | p-Cl | H | p-O-propargyl | 0.66 +/− 0.26 |
| 19 | CH | o-NO2 | p-Cl | H | p-O-propargyl | 0.29 +/− 0.35 |
| 20 | CH | o-NH2 | p-Cl | H | p-O-allyl | 0.62 +/− 0.16 |
| 21 | CH | o-NH2 | p-Cl | H | p-O-propargyl | 0.38 +/− 0.42 |
| 22 | N | o-Cl | m-Cl | p-Cl | H | 0.53 +/− 0.28 |
| 23 | N | o-Cl | p-Cl | H | H | 0.37 +/− 0.13 |
| 24 | N | o-NO2 | p-Cl | H | H | 8.25 +/− 0.13 |
| 25 | N | o-NO2 | p-OAc | H | H | 0.35 +/− 0.41 |
