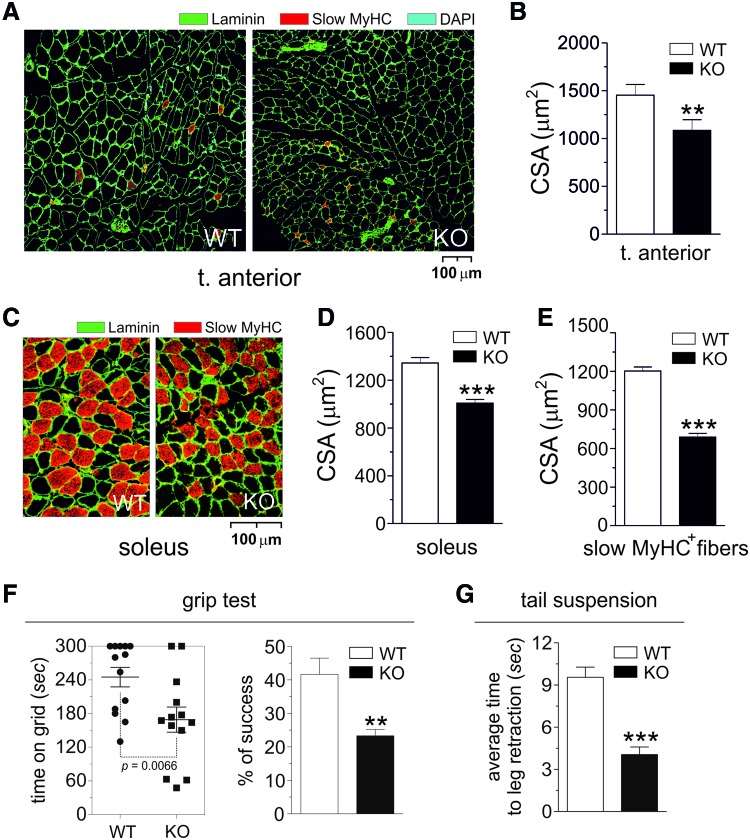
FIG. 2.
Size and composition of myofibers of skeletal muscle from GSNOR-KO mice. (A) Representative fluorescence microscopy images of tibialis anterior sections from 2-month-old GSNOR-KO (KO) and wild-type (WT) mice on staining with anti-laminin, anti-slow myosin heavy chain (slow MyHC), and DAPI to highlight myofibers boundaries, oxidative fibers, and nuclei, respectively. (B) Quantification of fiber cross-sectional area (CSA) in tibialis anterior from KO and WT mice. Results shown are the means±SEM of n=6 animals for each group. **p<0.01 calculated with regard to WT. (C) Representative fluorescence microscopy images of soleus sections from KO and WT mice on staining with anti-laminin and anti-slow MyHC to highlight myofiber boundaries and oxidative fibers, respectively. Scale bar: 100 μm (A, C). Quantification of total (D) and slow MyHC-containing (E) fiber cross-sectional area (CSA) in soleus from KO and WT mice. Results shown are the means±SEM of n=6 animals for each group. ***p<0.001 calculated with regard to WT. (F) Grip test: Evaluation of time that WT and KO mice gripped the grid by both hind and fore legs (left panel). Mice that gripped the grip within the cut-off time of 300 s and kept the grip for a further 10 s were recorded as a “success.” Percentage of success is reported in the right panel. Results shown are the means±SEM of n=33 animals divided into three different groups. **p<0.01 calculated with regard to WT. (G) Tail suspension test: Average time to the first episode of leg retraction. Results shown are the means±SEM of n=15 animals divided into three different groups and monitored thrice. ***p<0.001 calculated with regard to WT. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars