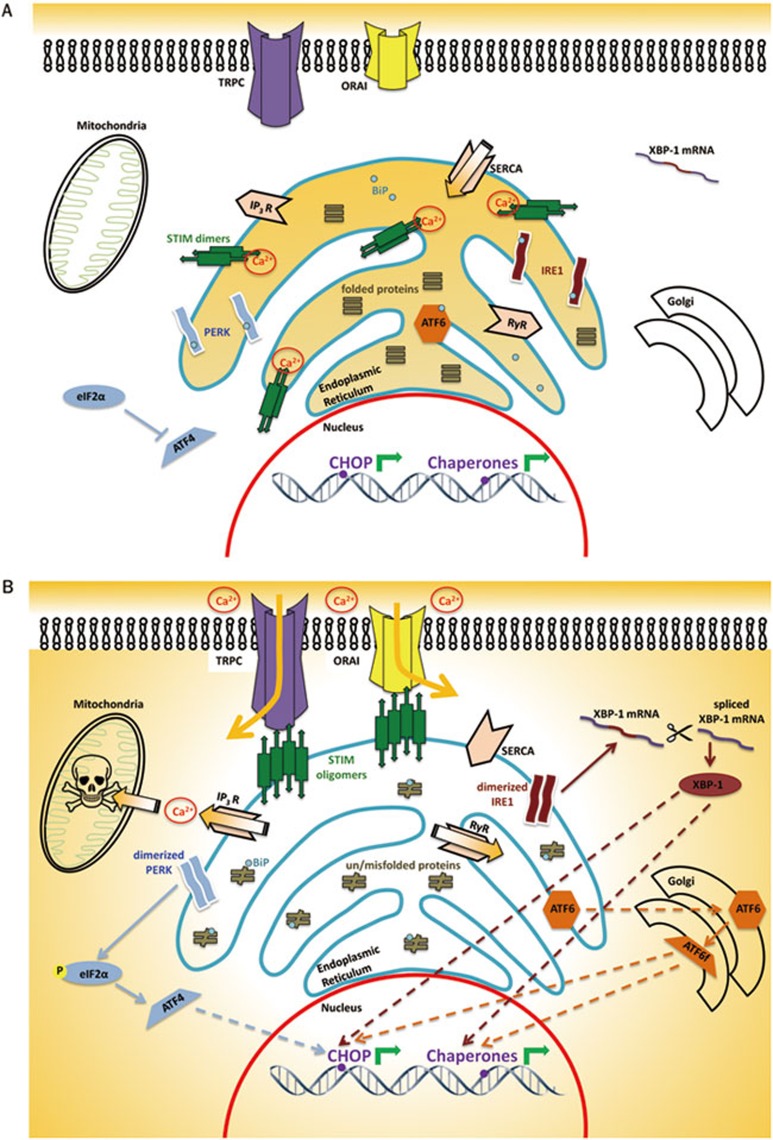
Figure 1.
(A) At physiological levels of luminal Ca2+, BiP remains bound to PERK, IRE1 and ATF6, suppressing their signalling activity. (B) Ischemia induced depletion of ER luminal calcium. ER protein folding capacity is exceeded causing competitive displacement of BiP from PERK, IRE1 and ATF6. Signalling pathways of the unfolded protein response are triggered when BiP dissociates from PERK, IRE1, and ATF6 allowing their dimerization and activation. In turn, transcription of CHOP and ER chaperones is upregulated. Release of ER Ca2+, particularly through IP3Rs, promotes uptake into mitochondria leading to mitochondrial injury and apoptosis. Store depletion further exacerbates the loss of cytosolic Ca2+ homeostasis through STIM-dependent signalling, possibly involving surface expressed Ca2+ permeable channels.