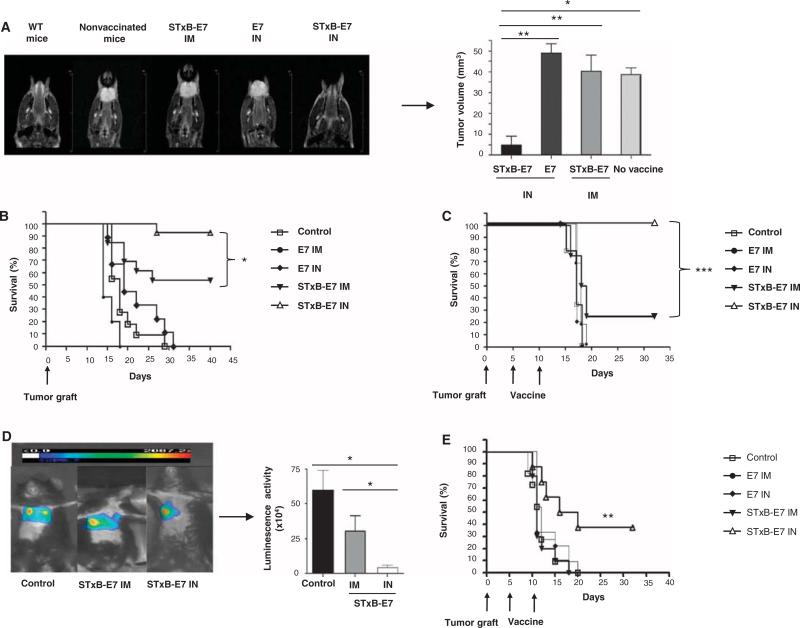
Fig. 2.
Intranasal immunization with STxB-E7 inhibits orthotopic head and neck and lung tumors in both prophylactic and therapeutic settings. (A and B) In a prophylactic setting, mice were immunized twice via the intranasal or intramuscular route with STxB-E7 or E7 peptide–based vaccines mixed with the aGalCer adjuvant. Seven days after the second vaccination, mice were grafted with 5 × 104 TC1 cells in the submucosa of the tongue. Representative MRI (coronal T2-weighted image of mouse tongue) (A, left) and tumor volume measured by MRI (A, right) at day 7 after tumor graft in the tongue are shown. (C to E) In the therapeutic setting, mice were immunized 5 and 10 days after grafting TC1 cells in the submucosa of the tongue (C) or 5 and 10 days after grafting TC1 luciferase (105 cells) into the lung (D and E), which was monitored by luminescence. Representative bioluminescence images of tumor-bearing mice in the lung are depicted in (D) (left panel). Luminescence (luciferase activity) in the chest was quantified 4 days after the second immunization with STxB-E7 via the intranasal route (n = 7) or the intramuscular route (n = 4) or in the nonvaccinated control mice (n = 4) (D, right panel). Kaplan-Meier survival curves of prophylactic (B) or therapeutic (C and E) cancer vaccine experiments for head and neck (B and C) and lung tumor (E) are shown. Each experiment was reproduced four times. *P < 0.05, **P < 0.01, ***P < 0.001.