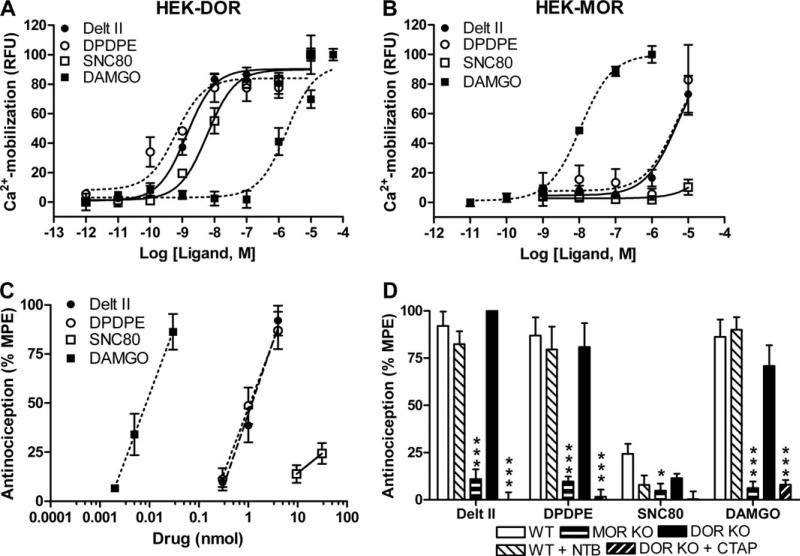
Figure 1.
Delta opioid receptor (DOR) selective agonists produce thermal antinociception via mu opioid receptors. HEK293 cells stably expressing murine DOR (A) or murine mu opioid receptors (MOR) (B) were transiently transfected with a chimeric Gαqi4-protein. Cells were stimulated with increasing doses of a DOR-selective (deltorphin II, [D-Pen2,D-Pen5]-Enkephalin [DPDPE], or SNC80) or MOR-selective (DAMGO) agonist and calcium mobilization was measured. Experiments were performed at least three times in triplicate, representative curves are shown. (C) Wild-type (WT) C57BL/6 mice (n = 8–10) were injected inrathecally with increasing doses of a DOR-selective or MOR-selective agonist and antinociception was measured using a radiant heat tail-flick assay. (D) WT, DOR knockout (KO), and MOR KO C57BL/6 mice (n = 8–12) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [4 nmol], SNC80 [30 nmol], or DAMGO [30 pmol]) and thermal antinociception was measured. In WT mice, the agonist response was unaffected by co-injection of the DOR antagonist Naltriben (.5 nmol). In DOR KO mice, the agonist response was inhibited by co-injection of the MOR antagonist CTAP (.2 nmol). Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100. Significance between groups was determined by analysis of variance followed by a Newman-Keuls post hoc analysis. *p < .05; ***p < .001. Delt II, deltorphin II; HEK, HEK293; MPE, maximal possible effect; NTB, Naltriben; RFU, relative fluorescence units.