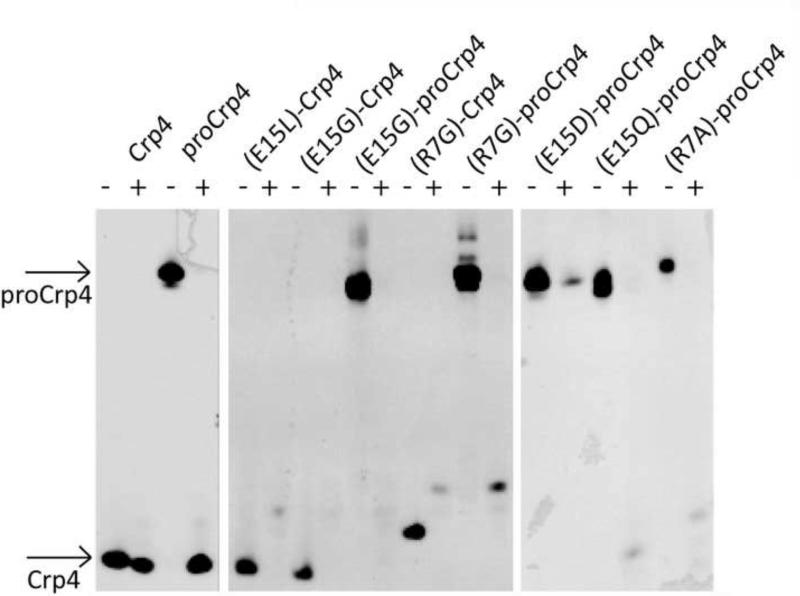
Figure 5.
Proteolytic stability of Crp4, proCrp4 and various salt-bridge mutated analogues. AU-PAGE analysis of untreated peptides (−) and peptides treated with trypsin. Arrows indicate the bands corresponding to Crp4 and proCrp4. Strikingly native proCrp4 is processed into a native-like form of Crp4, which is stable to further degradation. In contrast all mutant peptides, whether subjected to trypsin as precursors or mature peptides, are fully degraded by trypsin.