Abstract
Purpose
This study was conducted to investigate the effect of Se supplementation on prostate cancer incidence in men at high risk for prostate cancer.
Methods
A Phase 3 randomized, double-blind, placebo-controlled clinical trial was conducted in 699 men at high risk for prostate cancer (prostate specific antigen (PSA) >4 ng/ml and/or suspicious digital rectal examination and/or PSA velocity >0.75ng/ml/year), but with a negative prostate biopsy. Participants were randomized to receive daily oral placebo (N = 232), 200 µg selenium (N =234), or 400 µg selenium (N=233) as selenized yeast. They were followed every six months for up five years. The time to diagnosis of prostate cancer was compared between treatment groups using the Cox-proportional hazards model.
Result
Compared to placebo, the hazard ratios [95% confidence intervals] for risk of developing prostate cancer in the selenium 200 µg/day or the selenium 400 µg/day group were 0.94 [0.52, 1.7] and 0.90 [0.48, 1.7] respectively. PSA velocity in the selenium arms was not significantly different from that observed in the placebo group (p=0.18 and p=0.17, respectively).
Conclusion
Selenium supplementation appeared to have no effect on the incidence of prostate cancer in men at high risk. In conjunction with results of other studies, these data indicate that selenium supplementation may not have a role in prostate cancer chemoprevention.
Introduction
The American Cancer Society estimates 241,740 new diagnoses and 28,170 deaths due to prostate cancer in 2012, making it the most commonly diagnosed non-skin cancer (28%) and the second-most common cause (11%) of cancer related mortality among US men[1]. Nutritional strategies such as dietary selenium supplementation have been investigated for prostate cancer prevention. The Nutritional Prevention of Cancer study (NPC) was a Phase 3, randomized, double-blind, placebo-controlled clinical trial of 1312 men and women that was designed to investigate the effect of selenium supplementation on recurrence of non-melanoma skin cancer (NMSC) [2]. Although selenium did not reduce the incidence of NMSC, secondary analyses suggested reductions in prostate, lung, and colon cancer incidence associated with selenium supplementation [3]. Consequently, a number of studies were initiated to assess the effect of different forms of selenium on various stages of prostate cancer[4],[5],[6],[7]. The Negative Biopsy Trial (NBT) was a phase 3, randomized, double-blind, placebo-controlled clinical trial, initiated to investigate the effect of selenized yeast supplementation on incidence of prostate cancer. A secondary aim of the NBT was to determine whether selenium supplementation inhibits biochemical progression of prostate cancer as measured by changes in serum PSA measurements over time (PSA velocity).
Methods
Eligibility and study population
Detailed methodology of this trial has been described in an earlier publication[5]. Briefly, this is a randomized, double-blind, placebo-controlled, multi-center, Phase 3 clinical trial designed to investigate the effects of two doses of high-selenium yeast (200 or 400 µg/day) versus placebo on the incidence of prostate cancer. Subjects were followed every six months for up to five years. Subjects had to be < 80 years of age with one or more of the following: PSA >4ng/ml, DRE suspicious for prostate cancer, or PSA velocity >0.75ng/ml/year. All subjects had a prostate biopsy negative for cancer.
Randomization and recruitment
Subjects were recruited from urology offices at 20 sites in the United States and New Zealand Participants with adequate adherence to the protocol (80% or more pills taken during a 30 day run-in period) were randomized to receive placebo (N = 232), selenium 200 µg/day (N =234), or selenium 400 µg/day (N=233). Treatment group assignments were stratified based on study clinic and ethnicity. Subjects were followed every six months for up to up to five years. For subjects in the US, participation was complete at five years, whereas subjects in New Zealand received intervention for no more than three years.
Study agent and adherence
High-selenium yeast was provided by Cypress Systems (Fresno, CA) [2]. The study agent (two doses) and matched placebo caplets were coated with titanium oxide to ensure identical appearance, weight, taste and smell.
Biological sample collection and processing
Blood was drawn at baseline and at each subsequent visit to analyze complete blood count, plasma selenium concentration and PSA. Serum samples were separated into aliquots and frozen at −80°C for future research. At each visit, questionnaires were administered to obtain demographic characteristics, medical history, selenium toxicity information, and urological symptoms to verify eligibility. Tissue samples from the subject’s qualifying biopsy were requested from the subject’s physician and compiled in a biospecimen repository.
Total selenium concentration was measured by automated electrothermal atomic absorption spectrophotometry using a Perkin-Elmer Zeeman 3030 instrument (Norwalk, CT). Serum PSA levels were measured using the Abbott tumor markers assay module on an IMX analyzer (Abbott Diagnostics, Abbott Park, IL). In March 2005, Abbott Diagnostics replaced the AxSYM-PSA assay with the Total AxSYM-PSA assay, a combined assay for free and bound PSA. A variable adjusting for this change in the PSA assay was used in all statistical models to account for any effects this change might have had on PSA velocity. Both assays were FDA-approved for use in the US.
Study Endpoint
The primary endpoint was the incidence of biopsy-proven prostate cancer over the course of the study. The secondary endpoint was the rate of change of PSA over time (i.e. PSA velocity) using biannual PSA measurements.
Evaluation of Adverse Events
Expected adverse events included brittle nails, brittle hair, garlic breath, and liver/kidney function test abnormalities. Based on prior observations, additional potential expected adverse events included cataracts, glaucoma, and non-melanoma skin cancers. Collection of adverse event data occurred at each study visit.
Data and Safety Monitoring
An external Data and Safety Monitoring Committee (DSMC) was established before study initiation. This committee was responsible for reviewing protocol amendments, consent forms, accrual and retention rates, adverse events, and data analysis reports. Based on recommendation from the DSMC, an interim analysis for futility was carried out by an external statistician using a conditional power approach. The focus of these analyses was to determine the probability of finding a statistically significant difference in time to occurrence of prostate cancer between placebo and the combined selenium arms if the study was continued as specified in the protocol. These analyses indicated that the probability (conditional power) that the trial would eventually reach the conclusion that the selenium treatment arms are significantly better than the placebo arm was very low. Hence the DSMC recommended that the trial be stopped before all participants completed the full intervention duration. The interim analysis for futility was based on a conditional probability approach, whereas the data analysis plan for the full study utilized the Cox proportional hazards model.
Statistical analysis
The statistical analyses for this trial used the intention-to-treat principle. The sample size estimate for this trial was based on a three-arm design. It was estimated that 700 participants would allow for detection of at least a 50% treatment effect with 90% power, significance level of 0.05 with a dropout rate approximately 5% per year. Standard survival analysis techniques were used for analysis of the primary end-point. Cox proportional hazards regression was used to determine if the incidence of prostate cancer in the selenium arms was statistically significantly different as compared to placebo after adjusting for potential confounders such as age at baseline, baseline PSA, and baseline selenium concentrations.
A mixed effects model with patient-level random effects was used to assess the effect of selenium on PSA velocity in the three treatment groups. This model allows random intercept and slope for individual subjects in the trial and accounts for correlated data due to repeated measures over time [8]. An interaction term between treatment group and the time variable was created to assess differences in PSA velocity between groups. PSA values were transformed using ln(PSA+1) in order to correct for non-linearity over time and to stabilize their variance. Models were adjusted for race, baseline selenium, baseline age, duration of subject on study, and type of assay used to estimate PSA. Back-transformation was used to estimate yearly PSA velocity. Analyses stratified by tertiles of baseline selenium were also performed to determine whether the effect of selenium supplementation differed by baseline selenium level. A significance level of 0.05 was used for main effects as well as interaction terms. Proportions of adverse events were compared across groups using Fisher’s exact and log-rank test. Analyses were conducted using Stata10 (StataCorp IC, College station, TX) and SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
Recruitment and Participant Characteristics
Figure 1 describes patient recruitment and randomization. Eight hundred and seventy-five men were recruited for this trial and 699 (79.9%) subjects were randomized to receive placebo (n = 232), 200 µg/day selenium (n = 234) or 400 µg/day selenium (n = 233). Two hundred and ninety-two subjects (41.8%) completed the trial, seventy-four (10.6%) reached the study endpoint (diagnosis of biopsy proven prostate cancer) and sixty-one (8.7%) were still receiving study agent when the trial was stopped by the DSMC. Neither treatment group was significantly different from placebo in terms of study completion or withdrawal. The median months of follow-up were 36.8 35.4, and 35.0 respectively (p = 0.31). Study dropouts percentage was 34.1%, 41.9% and 40.8% for placebo, 200 µg/day selenium group and 400 µg/day selenium group respectively (p=0.173). Baseline patient characteristics are displayed in Table 1. Treatment groups were statistically balanced across all baseline variables.
Figure 1.
Participant cohort distribution
Table 1.
Baseline descriptive statistics by treatment group
| Variable | Total population (N= 699) |
Placebo (N = 232) |
Se 200µg/day (N = 234) |
Se 400µg/day (N =233) |
p-value |
|---|---|---|---|---|---|
| Age (mean, SD, years) |
65.4 (7.7) | 65.5(7.4) | 65.2(8.0) | 65.5(7.7) | 0.84 |
| Baseline PSA (mean, SD, ng/ml) |
6.8(5.5) | 6.4(5.6) | 7.2(6.2) | 6.9(4.5) | 0.41 |
| Baseline Selenium (mean, SD, ng/ml) |
126.1(25.5) | 124.5(24.7) | 126.6(26.9) | 127.2(24.8) | 0.51 |
| Caucasians (n, %) |
577(83.5) | 192(84.2%) | 195(83.7%) | 190(82.6%) | 0.90 |
Note: To calculate p-value, ANOVA was used for continuous variables and chi-square analysis was used for categorical variables.
Effects of selenium on incidence of prostate cancer
Mean adherence for the three study groups was 92.1%, 93.2% and 91.2% respectively (p = 0.098). Subjects achieved steady state concentrations of selenium in a dose-dependent manner within the first year (Supplemental figure 1). Twenty six (11.3%), 24 (10.3%) and 23 (10%) subjects reached the study endpoint (biopsy proven prostate cancer) in the placebo, Se 200 µg/day and Se 400 µg/day treatment groups, respectively (p=0.88). Mean and standard deviation of Gleason sum score was 6.7(0.9), 6.6(1.2) and 6.3(1.2), respectively (p=0.53). Time to study endpoint was not statistically significantly different in the two selenium groups versus placebo after adjusting for age, plasma selenium concentration, and serum PSA at baseline (Figure 2). None of the baseline variables modified the effect of selenium on the primary endpoint.
Figure 2.
Development of prostate cancer by treatment group. Placebo (solid line), Se 200 µg/day (dotted line ) and Se 400 µg/day (dashed line). Hazard ratio adjusted for age at baseline, race, baseline PSA, and baseline selenium plasma concentration. PCa = prostate cancer
Effect of selenium supplementation on PSA velocity
After adjusting for baseline selenium concentration, baseline PSA, baseline age, race, and type of PSA assay; the PSA velocities for the 200 and 400 µg/day treatment groups did not differ significantly from placebo (Table 2). Analyses stratified by tertiles of baseline selenium also yielded similar results, indicating that baseline serum selenium did not have a differential effect on PSA trajectory.
Table 2.
Estimated PSA velocities (ng/ml/yr) at 1yr intervals
| Placebo | 200µg/day | 400 µg/day | |
|---|---|---|---|
| End of year 1 | −0.006015 | 0.0917 | −0.0867 |
| End of year 2 | −0.006009 | 0.0930 | −0.0855 |
| End of year 3 | −0.006003 | 0.0944 | −0.0844 |
| End of year 4 | −0.005996 | 0.0958 | −0.0832 |
| End of year 5 | −0.005991 | 0.0971 | −0.0821 |
| p-value for difference in |
Ref | 0.18 | 0.17 |
| PSA trajectory vs placebo |
Effect of change in biopsy technique on detection of primary study endpoint
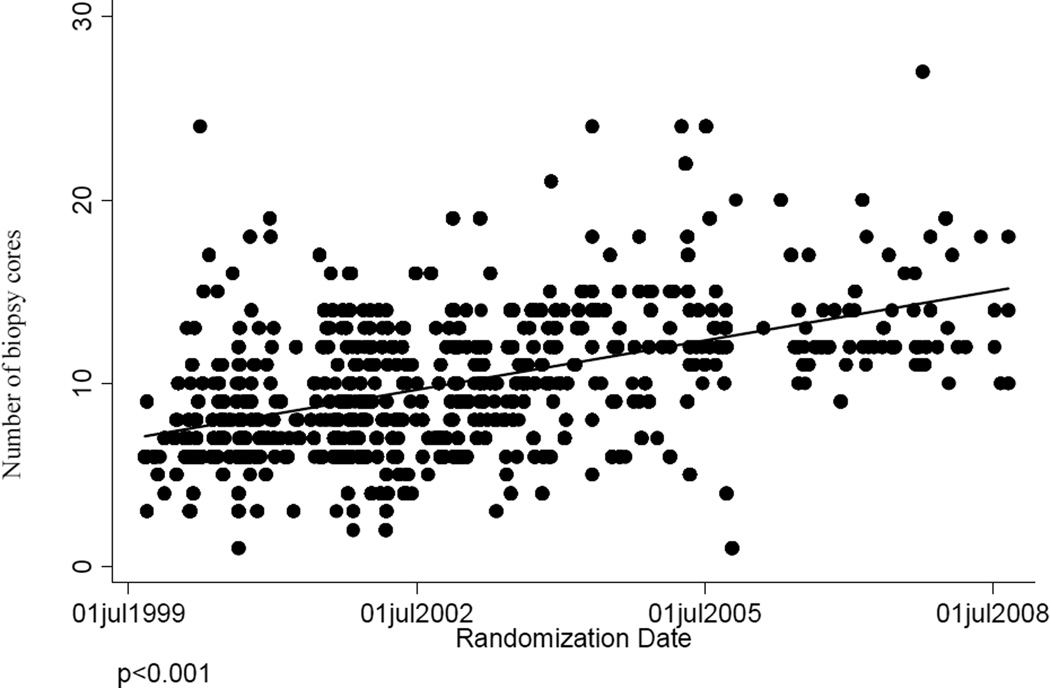
During the course of this trial, standard practices in the urology community for core biopsies changed throughout the US from a standard 6-core biopsy to a more invasive technique that collected additional cores. Data indicate that this change occurred early in the trial and continued to trend upward as the trial progressed (Figure 3). If the period during which the trial was conducted is divided into three tertiles, the median number of biopsy cores taken during each biopsy in the first, second and third tertile were 8, 9 and 12 respectively (p<0.001). This change had an impact on the detection of prostate cancer, presumably reducing the false negative rate of the 6-core biopsy. In men with biopsies of six cores or less, the rate of detecting prostate cancer on subsequent biopsy was 16.8%, whereas in men with biopsies having more than six cores, the same rate was 9.3%.
Figure 3.
Temporal trend in numbers of cores collected during prostate biopsy.
Out of a total of 699 men enrolled in this trial, 119 (17%) had baseline biopsies conducted with six or fewer cores. These were evenly distributed across the three treatment groups (38, 39 and 42 respectively). The rate of subsequent diagnosis of prostate cancer was 23.7%, 15.4% and 11.9%, respectively, in the three treatment groups but these differences were not statistically significant (p=0.36) and did not change upon restricting the analysis to placebo vs. any selenium (p=0.17). The hazard ratios for the 200 and 400 µg/day group did not achieve statistical significance [(Hazard ratios (95% confidence intervals): 0.65 (0.22, 1.86) and 0.48 (0.44, 1.6) respectively)]. The trend was also not statistically significant (p=0.23). Adjustment of this above model for age, race, baseline selenium concentration and baseline PSA did not affect the result.
Adverse Events
Of the 699 randomized participants, there were five deaths (2.2%) in the placebo group, three deaths (1.3%) in the 200 µg/day selenium treatment group, and two deaths (0.9%) in the 400 µg/day selenium treatment group (p = 0.45, Table 3). None were related to study treatment. With respect to grade 3 or 4 adverse events, there were 43 (18.6%) in the placebo group, 45 (19.2%) in the 200 µg/day group, and 39 (16.8%) in the 400 µg/day group (p = 0.78) (supplemental figure 2). Time to onset of the first grade 3 or 4 adverse event was the same in all treatment groups (p = 0.79). Three (1.3%) new cancers (excluding prostate and skin cancers) were diagnosed in the placebo group, two (0.9%) in the 200 µg/day, and three (1.3%) in the 400 µg/day selenium treatment group (p = 0.83). No significant differences were seen in the incidences of cataract/glaucoma or in hair/nail changes in the three treatment groups. Results for additional endpoints are presented in supplemental table 1.
Table 3.
Adverse events by treatment group
| Group | Placebo (N=232) |
200 µg/day (N=234) |
400 µg/day (N=233) |
p-value |
|---|---|---|---|---|
| Death (%) | 5 (2.2) | 3 (1.3) | 2 (0.9) | 0.45 |
| Other cancer (%) | 3 (1.3) | 2 (0.9) | 3 (1.3) | 0.83 |
| Cataract/glaucoma (%) | 13 (5.6) | 14 (6.0) | 15 (6.4) | 0.96 |
| NMSC (%) | 3 (1.3) | 8 (3.4) | 3 (1.3) | 0.20 |
| Brittle nail and hair (%)* | 26 (11.2) | 24 (10.3) | 20 (8.6) | 0.63 |
| Garlic breath, liver/kidney abnormality (%)* |
14 (6.0) | 13 (5.6) | 11 (4.7) | 0.82 |
exclude events with attribute less than 3.
Discussion
Results from this randomized, double-blind, placebo-controlled, multi-center, Phase 3 clinical trial showed no effect of selenium supplementation with either 200 or 400 ug/day of high-selenium yeast on the incidence of prostate cancer. Selenium supplementation had no statistically significant effect on PSA velocity, a clinical marker commonly used to monitor men at risk of prostate cancer.
These data are consistent with the findings of the largest prostate cancer prevention study conducted to date, the Selenium and Vitamin E Cancer Prevention Trial (SELECT). SELECT was a Phase 3, randomized, double-blind, placebo controlled prostate cancer prevention trial 200 µg/day selenomethione and vitamin E (400 IU/day), given individually or in combination in 35,533 men with PSA values ≤ 4 ng/ml. Study supplements were discontinued after a median follow-up of 5.46 years following a planned interim analysis showing no evidence of benefit and little likelihood of benefit with additional follow-up [9]. The primary finding of SELECT, strongly refutes the findings of the NPC study, which provided a large part of the rationale for the current, Negative Biopsy Trial [3]. Based on the strength of the findings of SELECT, the most reasonable conclusion is that selenium does not reduce the risk of prostate cancer. However, the possibility remains that the composition of the cohorts with respect to their selenium status might have contributed to the disparity between the results of the NPC and those of SELECT and the NBT. Results of SELECT study, differed from those of the NBT study in three important aspects. First, SELECT utilized purified selenomethionine [9], whereas high-selenium yeast (containing multiple organic and inorganic forms of selenium) was used in the NPC study and in the present trial [10]. Second, SELECT was carried out in average risk men (prostate specific antigen (PSA) <4ng/ml and non-suspicious digital rectal exam (DRE), whereas, the NBT was carried out in men at increased risk for prostate cancer [9]. Third, the NBT included participants from New Zealand (a region known for selenium deficiency), resulting in a wider range of baseline selenium levels than generally seen in the U.S. today. In the NPC study, mean baseline selenium plasma concentration was 115 ±22 ng/ml whereas the mean baseline selenium plasma concentration for the current study 126.1± 25.1 ng/ml. The baseline selenium plasma concentration of men in the NBT study was statistically significantly higher than that measured in the NPC study (p<0.0001). Additionally, each tertile of baseline selenium in NBT was statistically significantly higher than the corresponding tertile in NPC (Table 4). In the NPC study, the protective effect was noted only in subjects with baseline selenium <123 ng/ml [11]. Fifty-five percent of the subjects in the NBT have baseline selenium > 123 ng/ml. Supplementation of selenium has increased dramatically in diets of human [12] and livestock [13] since the 1980s (when the NPC enrollment occurred) and may help potentially explain the shift in baseline. Currently, selenium deficiency in the United States is rare, but it is seen in other countries, most notably in China, where the concentration of selenium in soil is low [14]. According to The Centers for Disease Control and Prevention, values less than 70 ng/mL or 0.8 µmol/L are indicative of selenium deficiency [14]. Based on data obtained from the National Health and Nutrition Examination Survey (NHANES) III (1988–1994), diets of most Americans provide the necessary amount of selenium [15]. The mean and median serum selenium plasma concentrations in NHANES III were 1.58 µmol/L and 1.56 µmol/L, respectively. In NHANES 2001–2002, less than 3 percent of survey participants had a dietary intake of selenium below the estimated average requirement [14].
Table 4.
Comparison of baseline selenium plasma concentration tertiles between the Negative Biopsy Trial and the previous Nutritional Prevention of Cancer study
| NBT | NPC | p-value | |||
|---|---|---|---|---|---|
| Tertile | N | (ng/ml, mean (SD)) | N | (ng/ml, mean (SD)) | |
| 1 | 230 | 101.1(14.7) | 317 | 92.4(11.1) | <0.001 |
| 2 | 230 | 126.1(4.7) | 305 | 114.6(5.0) | <0.001 |
| 3 | 229 | 151.1(21.5) | 305 | 138.4(16.0) | <0.001 |
| Total population |
681 | 126.1(25.5) | 927 | 115(22.0) | <0.001 |
During the course of the trial, 74 subjects were diagnosed with prostate cancer despite having a prostate biopsy negative for cancer at the beginning of the study. For a chronic disease such as prostate cancer, it is unlikely that the disease developed to a detectable stage during a span of five years (median follow-up 36 months). It is more likely that some of the cancer foci were missed during the initial biopsy leading to a false negative result. Since the distribution of false negative results cannot be determined, it is unclear how this could affect the results of the present study.
Another important factor to consider when analyzing these data was the temporal change in prostate biopsy technique. When this trial was initiated in 1999, ultrasound guided transrectal sextant biopsy was the standard prostate biopsy technique for a prostate biopsy[16]. Around the year 2000, papers examining improved efficacy with increased numbers of cores and different techniques (laterally directed biopsies) indicated promising results[17],[18],[19],[20],[21]. This led to changes in biopsy techniques moving from a standard of 6 to a standard 12-core biopsy. Saturation biopsies, having as many as 23 to 31 cores, were also proposed for men who had repeated negative biopsies but high PSA [22],[18]. False negative rates for the sextant biopsy technique ranged from 20–23%[17],[23], thus this was used as the event rate for estimating power and for calculating sample size for this study when it was originally designed. As the number of biopsy cores increased, the false negative rate dropped to approximately 11%, which reduced the event rate (detection of prostate cancer on subsequent biopsy). Thus, the ability to detect differences in this study was adversely affected.
In men in whom the baseline biopsy consisted of six cores or less, the incidence of prostate cancer in the selenium supplemented groups as compared to placebo (23.7% vs. 15.4% and 11.9%) was not statistically significant (p=0.36). Additionally, in this subset a Cox proportional regression analysis indicated no statistically significant difference in the time to prostate cancer diagnosis in the two selenium arms as compared to placebo (HR(95%CI): 0.65(0.22, 1.86) and 0.48(0.14, 1.60) respectively). While the number of biopsy cores did not affect the selenium-associated outcomes in this study, these results highlight the difficulties associated with conducting a large long-term Phase 3 chemoprevention trial in a dynamic and changing field.
Supplementary Material
Acknowledgments
This manuscript is dedicated to all the participants who volunteered for this study. We thank all the physicians and staff at all the sites for patient recruitment and participation. (University of Arizona, Tucson, AZ; University of North Carolina, Chapel Hill, North Carolina; Midwest Prostate/Urology Healthcenter, Chicago, Illinois; Stanford University, Stanford, CA; Aegis Medical Associates, Annapolis, MD; University Hospitals of Cleveland, Cleveland, Ohio; Urological Associates of Southern CA, Fresno, CA; Louisiana State University, Shreveport, LA; Overton Brooks VA Medical Center, Shreveport, LA; Sun Health Research Institute, Phoenix, AZ; University of New Mexico, Albuquerque, NM; University of Arkansas, Little Rock, AK; Thomas Jefferson University, Philadelphia, PA; Roswell Park Cancer Institute, Buffalo, NY; Carle Cancer Center, Urbana, IL; University of Oklahoma, Oklahoma City, OK; Auckland Hospital, Auckland, New Zealand; and Waikato Urology Clinic, Waikato, New Zealand). We thank Dr. Chengcheng Hu for performing the interim analysis and members of the DSMC (Stephen George, Ph.D., Timothy Byers, M.D., M.P.H., Harvey Cohen, M.D., Ph.D., Robert Greenberg, M.D.) for their guidance and oversight regarding protection of human subjects. This work was supported by grants from the National Cancer Institute (PHS CA077789 & PHS 023074). We thank Dr. Michael Jameson (Waikato, New Zealand) for his substantial contributions in conceptualizing and initializing the trial in New Zealand.
References
- 1.ACS, Cancer Facts & Figures. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Clark LC, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276(24):1957–1963. [PubMed] [Google Scholar]
- 3.Duffield-Lillico AJ, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11(7):630–639. [PubMed] [Google Scholar]
- 4.Stratton MS, et al. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev Res (Phila) 3(8):1035–1043. doi: 10.1158/1940-6207.CAPR-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton MS, et al. Selenium and prevention of prostate cancer in high-risk men: the Negative Biopsy Study. Anticancer Drugs. 2003;14(8):589–594. doi: 10.1097/00001813-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 7.Algotar AM, et al. Dose-dependent effects of selenized yeast on total selenium levels in prostatic tissue of men with prostate cancer. Nutrition and Cancer. 2011 doi: 10.1080/01635581.2010.516476. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svatek RS, et al. Critical analysis of prostate-specific antigen doubling time calculation methodology. Cancer. 2006;106(5):1047–1053. doi: 10.1002/cncr.21696. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark LC, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81(5):730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 11.Duffield-Lillico AJ, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91(7):608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 12.NIH State-of-the-Science Conference Statement on Multivitamin/Mineral Supplements and Chronic Disease Prevention. NIH Consens State Sci Statements. 2006;23(2):1–30. [PubMed] [Google Scholar]
- 13.FDA Cf.V.M. CVM update. Rockville, MD: U.S.F.a.D. Administration, Editor. 1997, U.S Food and Drug Administration; Selenium Regulations Finalized, in. [Google Scholar]
- 14.Sciences NCf.E.H.D.o.L., editor. CDC. National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 1999–2002. Atlanta, Georgia: Center for Disease Control and Prevention; 2008. pp. 101–106. [Google Scholar]
- 15.Niskar AS, et al. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol Trace Elem Res. 2003;91(1):1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 16.Jones JS. Saturation biopsy for detecting and characterizing prostate cancer. BJU Int. 2007;99(6):1340–1344. doi: 10.1111/j.1464-410X.2007.06868.x. [DOI] [PubMed] [Google Scholar]
- 17.Presti JC, Jr., et al. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163(1):163–166. discussion 166-7. [PubMed] [Google Scholar]
- 18.Stewart CS, et al. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166(1):86–91. discussion 91-2. [PubMed] [Google Scholar]
- 19.Epstein JI, Walsh PC, Carter HP. Importance of posterolateral needle biopsies in the detection of prostate cancer. Urology. 2001;57(6):1112–1116. doi: 10.1016/s0090-4295(01)00979-7. [DOI] [PubMed] [Google Scholar]
- 20.Chang JJ, et al. Prospective evaluation of lateral biopsies of the peripheral zone for prostate cancer detection. J Urol. 1998;160(6 Pt 1):2111–2114. doi: 10.1097/00005392-199812010-00044. [DOI] [PubMed] [Google Scholar]
- 21.Norberg M, et al. The sextant protocol for ultrasound-guided core biopsies of the prostate underestimates the presence of cancer. Urology. 1997;50(4):562–566. doi: 10.1016/S0090-4295(97)00306-3. [DOI] [PubMed] [Google Scholar]
- 22.Borboroglu PG, et al. Extensive repeat transrectal ultrasound guided prostate biopsy in patients with previous benign sextant biopsies. J Urol. 2000;163(1):158–162. [PubMed] [Google Scholar]
- 23.Rabbani F, et al. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159(4):1247–1250. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.