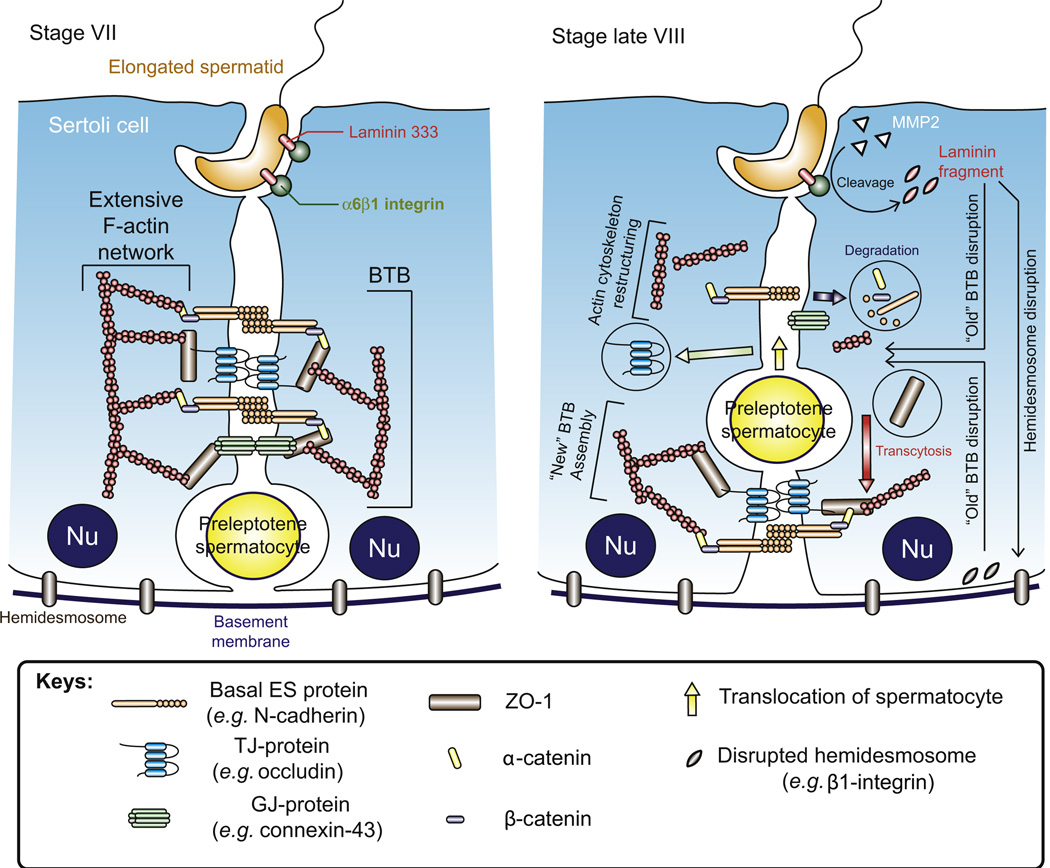
Figure 6.2. Restructuring of the BTB to facilitate the transit of preleptotene spermatocytes at stage VIII of the epithelial cycle.
Before BTB restructuring takes place, its integrity is maintained by coexisting TJs, basal ES and GJs which interact with each other and linked to actin cytoskeleton for structural support via adaptor proteins such as ZO-1. Besides, desmosome is also present at the Sertoli cell–cell interface at the BTB. On the other hand, elongated spermatids are also anchored to the Sertoli cell via a testis-specific apical ES protein complex in which laminin-333 residing at the elongating spermatid is linked to α6β1-integrin restricted to the Sertoli cell. At stage VIII of the epithelial cycle, when preleptotene spermatocytes are in transit at the BTB to enter the apical compartment for further development, the “old” BTB above the spermatocyte disassembles to “open” the BTB. This process is mediated by the apical ES–BTB–hemidesmosome functional axis, in which laminin 333 at the apical ES is cleaved by MMP2 to generate bioactive laminin fragments. The laminin fragments induce disruption of the “old” BTB and cause the loss of hemidesmosome function which also contributes to the “opening” of the “old” BTB. Besides, BTB restructuring is also facilitated by mTORC1 as well as by the reorganization of actin cytoskeleton mediated by actin-regulating proteins, such as the Arp2/3–N-WASP complex and Eps8 which induce a “branched/debundled” and “bundled” configuration of the actin filaments at the basal ES, respectively. Without the support from the dense F-actin network, BTB proteins are internalized through endocytosis and the internalized BTB proteins can either undergo degradation or being recycled for the assembly of “new” BTB via transcytosis at the base of the preleptotene spermatocytes. It is likely that molecules, such as testosterone, that promote BTB integrity may be working in concert with mTORC2 underneath the spermatocyte in transit to assemble a “new” BTB before the “old” BTB above the transiting spermatocyte is disassembled, so that the barrier function can remain intact during germ cell movement at the site. For color version of this figure, the reader is referred to the online version of this book.