Abstract
Considerable progress has been made over the past couple of decades concerning the molecular bases of neurobehavioral function and dysfunction. The field of neurobehavioral genetics is becoming mature. Genetic factors contributing to neurologic diseases such as Alzheimer’s disease have been found and evidence for genetic factors contributing to other diseases such as schizophrenia and autism are likely. This genetic approach can also benefit the field of behavioral neurotoxicology. It is clear that there is substantial heterogeneity of response with behavioral impairments resulting from neurotoxicants. Many factors contribute to differential sensitivity, but it is likely that genetic variability plays a prominent role. Important discoveries concerning genetics and behavioral neurotoxicity are being made on a broad front from work with invertebrate and piscine mutant models to classic mouse knockout models and human epidemiologic studies of polymorphisms. Discovering genetic factors of susceptibility to neurobehavioral toxicity not only helps identify those at special risk, it also advances our understanding of the mechanisms by which toxicants impair neurobehavioral function in the larger population. This symposium organized by Edward Levin and Annette Kirshner, brought together researchers from the laboratories of Michael Aschner, Douglas Ruden, Ulrike Heberlein, Edward Levin and Kathleen Welsh-Bohmer conducting studies with Caenorhabditis elegans, Drosophila, fish, rodents and humans studies to determine the role of genetic factors in susceptibility to behavioral impairment from neurotoxic exposure.
Keywords: Genetics, Behavioral neurotoxicology, C. elegans, Drosophilia, Zebrafish, Mice
1. Introduction
This symposium organized by Edward Levin and Annette Kirshner brought together researchers pursuing Caenorhabditis elegans, Drosophila, fish, rodent and human studies to determine the role of genetic factors in susceptibility to behavioral impairment from neurotoxic exposure studies. The laboratories of Drs. Michael Aschner, Douglas Ruden, Ulrike Heberlein, Edward Levin and Kathleen Welsh-Bohmer all contributed to this work.
Behavioral neurotoxicology has been instrumental in identifying and characterizing the functional consequences of neurotoxicants on the function of both experimental animals and humans. In the past, the search for mechanisms of behavioral toxicity focused mainly on neurochemical and neuropathological studies. Advances in molecular neurobiology have opened the way for determining genomic mechanisms of behavioral neurotoxicology. The availability of a variety of technological and methodological advances to aid in the development of appropriate animal models and systems will do much to move the field of behavioral neurotoxicology forward. Experts in behavioral genetics and behavioral neurotoxicology need to inform each other of the potential use that new methods, technologies and tools could have on the field of behavioral neurotoxicology in establishing a more mechanistic basis for the role of neurotoxic exposure in behavioral dysfunction.
Considerable progress has been made over the past couple of decades concerning the molecular bases of neurobehavioral function and dysfunction. The field of neurobehavioral genetics is becoming mature. Genetic factors contributing to neurologic diseases such as Alzheimer’s disease have been found and evidence for genetic factors contributing to other diseases such as schizophrenia and autism are likely. This genetic approach can also benefit the field of behavioral neurotoxicology. There is great potential to rapidly advance the field of behavioral neurotoxicology by accessing the tools of molecular biology both for discovery of mechanisms and for better understanding of individual differences in neurotoxic response. It is clear that there is substantial heterogeneity of response concerning the behavioral impairments from neurotoxicant exposure. Many factors contribute to differential sensitivity, but it is likely that genetic variability plays a prominent role. Important discoveries concerning genetics and behavioral neurotoxicity are being made on a broad front from work with invertebrate and fish mutant models to classic mouse knockout models and human epidemiologic studies of polymorphisms. Discovering genetic factors of susceptibility to neurobehavioral toxicity not only helps identify those at special risk, it also advances our understanding of the mechanisms by which toxicants impair neurobehavioral function in the larger population.
A symposium on the genetic aspects of behavioral neurotoxicology was conducted at the International Neurotoxicology Conference in Rochester, NY, October 13–17, 2008. The goal of the symposium was to highlight the advantage of diverse animal models to elucidate the genetic basis of behavioral neurotoxicology. Each of the presenters highlighted the benefits of their model in studying aspects of genetic alteration on behavior.
Dr. Aschner presented the utility of C. elegans in studying mechanisms of toxic effects on the nervous system. He cited the advantages of C. elegans, its small size (adults are ~1 mm long), ease of maintenance, speedy generation time (3 days), and large brood size (>300 progeny per hermaphrodite) for its use in cellular, molecular, and genetic analyses. In addition, its genome and biosynthetic and metabolic pathways are highly conserved with mammals. Additional advantages are the ability to assess, among other endpoints, the behavior and genetic effects from a variety of exposures including metals and pesticides. One can also model various human neurodegenerative disorders in C. elegans using either mutant strains or chemical exposure. C. elegans also lends itself to modern technological approaches such as high-throughput analysis, microfluidics, and quantitative trait locus mapping to identify relevant genes and behaviors.
Dr. Ruden presented the merits of studying the genetics and genomics of neurotoxicology in Drosophila melanogaster (D. melanogaster). There are thousands of wild type and mutant strains available in which hundreds of developmental and behavioral mutations have been identified along with homologous genes for over 70% of known human disease genes. This availability allows for very sophisticated techniques to knockout, reduce or overexpress almost any gene in the genome. In addition, quantitative genetics will permit in D. melanogaster, deep sequencing, a newer analytic technology which can characterize individually rare polymorphisms. This will allow the study of the role of normal genetic variation in phenotypic differences, the technology of which eventually can be used to identify a quantitative trait (drug response) useful in “personalized medicine”. Lastly, fruit flies are useful in identifying genes that may be resistant or sensitive to a toxicant exposure (Gene × Environment or G × E interaction) using a technique that he calls “genetical toxico genomics” which may have major implications in the role of natural variation in nervous system development in a toxic environment.
Dr. Heberlein expanded on the usefulness of the Drosophila model in which to study the genes and pathways that mediate acute and chronic behavioral responses to environmental exposure, in this case ethanol. She pointed out that multiple hypotheses have been presented to explain ethanol-induced brain damage. The mechanisms proposed vary from the consequences of thiamine deficiency to the production of reactive oxygen species (ROS) and increased production of polyamines depending on cell type and developmental stage to explain the types of damage induced. Finally, ethanol is known to bind to N-methyl-D-aspartate (NMDA) and it is thought that this interaction may explain many of the drug’s neurotoxic effects. Using flies, they have shown that acute ethanol exposure leads to widespread cell death in the antennae, the primary olfactory organs of flies. Ethanol-induced death of Drosophila olfactory neurons is apoptotic in nature, requires shaggy (sgg), the Drosophila homolog of GSK-3β, can be prevented by treatment with the GSK-3β inhibitor LiCl, and can be blocked by electrical silencing of the olfactory neurons, demonstrating that ethanol-induced death in these cells is due to excitotoxicity, requires NMDA receptors in the olfactory neurons, and that sgg and the NMDA receptor are likely acting in concert to mediate this effect. They hope to use their model for ethanol-induced neuronal cell death to identify genes and mutations involved in sensitivity to ethanol neurotoxicity allowing a greater understanding of the molecular processes of neuronal death, which is seen in alcoholic dementia.
Dr. Levin and co-workers have used zebrafish and rodent models to investigate the behavioral neurotoxicology of environmental toxicants. Primarily, they have concentrated on toxic effects on cognitive function and other aspects of behavioral plasticity. Zebrafish is the piscine model most widely used to study the molecular bases of development in general and neurodevelopment in particular. Their clear chorion and reporter systems allow continuous visualization of developmental processes. The variety of mutant models and the availability of morpholinos in which parts of the genome can be reversibility suppressed during early development provide ways to test the role of genetic factors in neurodevelopment. The Levin lab and others have developed a variety of behavioral tests to provide assessment of the functional consequences of neural impairment. Their behavioral tests assessing spatial learning and memory detected the persisting impairment caused by early developmental exposure to low doses of the pesticide chlorpyrifos. Chlorpyrifos also caused significant hyperactivity in a rapid test of motor reaction to a tactile startle. Chlorpyrifos-induced behavioral impairment have been related to alterations in neurochemical indices of dopamine and serotonin neurotransmitter systems in zebrafish.
Levin et al. have also worked with the classic mouse knockout model for testing genetic influences on behavior. In particular they have used metallothionein 1 and 2 knockout mice and tested the interactions with developmental exposure to mercury. Metallothionein 1 and 2 knockouts themselves have cognitive impairment. They also potentiate the persisting learning impairment caused by early postnatal mercury exposure at a dose that does not affect wild-type control mice. Metallothionein × mercury interactions in dopamine levels that were detected may be important in explaining the differential response to mercury in terms of cognitive function.
Dr. Welsh-Bohmer discussed the possible gene × environment interaction in Alzheimer’s disease (AD) in human studies. While one of the most influential risk factors for AD is inherited disease susceptibility factors, genes may not be solely responsible for AD risk and symptom onset in most cases of AD. Some of the more speculative risk factors for AD are environmental toxicants, such as pesticides, organic solvents, air-borne pollutants, and heavy metals, have been linked to a number of neurological disorders including Parkinson’s disease and multiple sclerosis. Since few studies have been conducted as to these neurotoxicants’ significance in AD, the risk relationships of these exposures are not well established. However, the epidemiological work being conducted in the Cache County Memory Study (CCMS) in an aging population, examines both the genetic and environmental (pesticides) contributions to AD risk and their effects on the expression of the disease. While the study cannot distinguish between classes of pesticides, the organophosphates and organo-chlorines were the most commonly reported pesticide exposures in those who developed incident dementia.
These presentations highlight the advantages of studying a variety of species to determine genomic mechanisms of behavioral neurotoxicity.
Caenorhabditis elegans in neurotoxicology
Michael Aschner and Kirsten Helmcke
Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, USA
Experimental models that allow live in vivo analysis with resolution of single neurons offer a great advantage in providing insight into mechanism/s of neuronal injury. There is also increased need for reproducible high-throughput approaches that address the interactions between genetic mutations and environmental insults on biological networks. The extraordinary conservation of both genetic elements and differentiation processes between mammals and non-mammalian organisms have led to the use of Caenorhabditis elegans (C. elegans) in deciphering mechanisms of neurotoxicity. Its genome and biosynthetic and metabolic pathways are highly conserved with mammals, yet the small size (adults are ~1 mm long), ease of maintenance, speedy generation time (3 days), and large brood size (>300 progeny per hermaphrodite) allow for a nearly limitless supply of worms for cellular, molecular, and genetic analyses. This allows for the dissection of operative neurotoxic mechanisms as well as high-through put studies on changes associated with chemical insults. The transparency of the worm and the ease of making reporter gene fusions further facilitate visualization of neuronal morphology and protein expression patterns within the living nervous system. Moreover, the availability of a complete three-dimensional map of the 302-cell nervous system allows for the identification of most synapses between neurons (Hobert, 2005). The self-fertilizing hermaphrodite permits quick and easy homozygosity of mutations; males can be used to set up crosses of strains with different genetic backgrounds, allowing for the generation of lines with multiple mutations. The completed sequence of the genome, the ability to perform whole animal PCR and the existence of a high-density polymorphism map of a related strain of the wild-type C. elegans allows for quick and easy mapping of mutations within practically any gene. Gene knockdowns can be generated with RNAi techniques and loss-of-function mutants can be produced by site-directed mutagenesis.
C. elegans was originally developed as an experimental model to study nervous system development (Brenner, 1974). Its nervous system has been systematically investigated and differentiation and migration patterns have been described (Sulston, 1983; Sulston et al., 1983). Furthermore, the main neurotransmitter systems in this organism are phylogenetically conserved thus permitting the analysis of changes in neurotransmitter expression in response to assorted variables, including mutations and toxic insults. In addition, knowledge gained on the C. elegans life-cycle and behavior facilitates the use of these animals in understanding the developmental and behavioral effects of physical and chemical interventions, including neurotoxicants. C. elegans has been effectively used in aging research, and has elucidated pathways involved in increased longevity through the discovery of mutant strains with significant increases in lifespan (Arantes-Oliveira et al., 2003) and the ability of mild heat shock to induce an increased lifespan (Cypser et al., 2006). Several well-characterized behaviors of C. elegans have been studied, and these (thrashing, pharyngeal pumping, mating and others) can be easily evaluated with computerized video tracking systems. Numerous studies have elucidated effects of toxicants on locomotion, including a decrease in body bends and head thrashes following exposure to various metals (Xing et al., 2009) and pesticides (Ruan et al., 2009), and alterations in feeding and reproduction after exposure to neurotoxicants (Boyd et al., 2009).
Toxicity of various types of toxicants, including metals and pesticides, has been studied in C. elegans and has been evaluated as a model for various neurodegenerative disorders (Berkowitz et al., 2008; Silverman et al., 2008). The extensive advantages of using a C. elegans model system have given researchers the capability of examining various endpoints, many of which assess nervous system functioning. These include examining the morphology, behaviors, and gene expression of the C. elegans nervous system. Importantly high-throughput approaches can be applied to these lines of experimentation, using such tools as computer tracking software to assess behavior and biosorters and microfluidics to manipulate large numbers of worms quickly, efficiently, and precisely (Hulme et al., 2008; Peterson et al., 2008).
The toxicity of many metals including aluminum, arsenic, barium, cadmium, copper, lead, mercury, uranium, and zinc has been investigated using the C. elegans model system, looking for behavioral and morphological alterations upon toxicant exposure (Leung et al., 2008). Behavioral tests can be particularly enlightening when using the C. elegans model due to the extensive body of knowledge describing specific circuits responsible for specific behaviors. For example, the pharyngeal nervous system of C. elegans is a circuit of its own including M3, M4, MC, NSM, RIP, and I neurons (Larsen et al., 1995). Therefore, the decrease in feeding noted when C. elegans were exposed to cadmium or mercury (Boyd et al., 2003; Jones and Candido, 1999) indicates that there may be alterations in this circuit, which could direct investigators’ efforts to examine the involvement of specific neurons in this effect.
Pesticides, such as paraquat, rotenone, and organophosphates, have also been assessed in the C. elegans model, revealing novel neurotoxic mechanisms through which these agents exert their effects (Leung et al., 2008). While studying paraquat toxicity, several strains have been generated that have either increased (Ishii et al., 1990) or decreased (Fujii et al., 2005) sensitivity to the herbicide. The identities of many of the proteins encoded by the genes that cause these alterations are unknown and the knowledge about their involvement in paraquat toxicity is forthcoming. However, researchers have found that alterations in antioxidants such as superoxide dismutase can cause alterations in the response of C. elegans to paraquat. More specifically, mutants that lack these enzymes display increased sensitivity (Yang et al., 2007) while those that overexpress these enzymes display decreased sensitivity (Yanase et al., 2002; Burmeister et al., 2008).
Models for various neurodegenerative disorders have been elucidated and exploited in C. elegans, including Duchenne muscular dystrophy (DMD), Parkinson’s disease (PD), Huntington’s disease (HD), and Alzheimer’s disease (AD). For example, the DYSTROPHIN gene, responsible for DMD, is conserved in C. elegans and study of mutants has revealed progressive muscle degeneration (Gieseler et al., 2000). Parkinson’s Disease is mimicked by C. elegans exposed to 1-methyl-4-phenylpyridinium (MPP+) (Braungart et al., 2004) or 6-hyrdroxydopamine (6-OHDA) (Nass et al., 2002), resulting in damage to the dopaminergic nervous system. The understanding of Huntington’s disease has been increased by using C. elegans. By expressing polyQ Huntingtin variants in C. elegans, discoveries have been made revealing genes interactions with Huntingtin as well as axonal defects and protein aggregation (Parker et al., 2001; Holbert et al., 2003). The investigation into AD has been advanced using mutant worms and transgenic strains and these have helped to elucidate the mechanisms of disease progression. By expressing human APP Abeta (1–42) or TAU in C. elegans, researchers were able to gain insights into disease progression and the involvement of other genes (Boyd-Kimball et al., 2006). Additionally, new potential therapeutics for AD have been tested using the C. elegans model, as it allows for relatively quick and easy drug screening in a full animal model as opposed to a cell line (Wu et al., 2006; Arya et al., 2009).
In summary, C. elegans is a model amenable to providing answers to a multitude of biological questions, including those that stem from investigations into toxicology and neurotoxicology. Investigators have been able to reveal toxicity mechanisms of numerous toxicants by exploiting the many advantages of the C. elegans model system. Extensive reviews of the studies using C. elegans as a toxicological model are available (Leung et al., 2008; Peterson et al., 2008). Understanding these mechanisms of toxicity will elucidate ways in which exposure to toxicants can be best remedied and various therapeutic solutions can be tested in a manageable fashion.
Drosophila melanogaster in neurotoxicology research
Douglas M. Ruden, Helmut V.B. Hirsch, Greg Lnenicka, Lang Chen, Grier P. Page, Debra Possidente, and Bernard Possidente
Department of Environmental Health Sciences, Wayne State University, Detroit, MI, USA
Drosophila melanogaster (D. melanogaster) is an ideal model to study the genetics and genomics of neurotoxicology. Its genetics has been studied and refined for almost 100 years and thousands of wild type and mutant strains are available from the stock centers (www.flybase.org). Homologs for over 70% of human disease genes are present in D. melanogaster, and those 30% that are not present can often be studied by ectopically expressing them in the whole organism or specific tissues (www.homophila.org). Classical studies of D. melanogaster have identified hundreds of developmental and behavioral mutations, and balancer chromosomes allow the maintenance of lethal alleles without selection (Lindsley and Zimm, 1992). Large transposon insertion screens have been performed that knockout or reduce expression of over 80% of D. melanogaster genes (Thibault et al., 2004). Also, small isogenic deficiencies (~100,000) have been generated that uncover over 90% of the unique sequences in the genome (Ryder et al., 2007). The GAL4-UAS 2-component expression system has been widely used in D. melanogaster such that almost any gene can be over-expressed in almost any tissue (or brain region) by crossing two strains together and analyzing their progeny (Duffy, 2002). The GAL4-UAS system has also been used to knockout nearly any gene in all tissues of more than 22,000 stains containing dsRNA under the control of the GAL4-UAS. These strains are available from the Vienna stock center (Dietzl et al., 2007).
D. melanogaster is also a powerful model for quantitative genetics. In standard quantitative trait locus (QTL) mapping experiments, one studies only one or a small number of traits at a time, such as bristle number or triglyceride levels (Falconer and Mackay, 1996; De Luca et al., 2005). Most QTL mapping experiments in D. melanogaster involve analyzing recombinant inbred lines (RILs) that were derived by inbreeding F2 offspring from two parental laboratory strains. For example, the “roo lines” were derived from Oregon R (ORE) and Russian 2B (2B), which have an estimated 600,000 sequence polymorphisms (SNP rate: ~1/250 nucleotides). New generation sequencing technologies will soon allow the re-sequencing of dozens or even hundreds of wild-type strains, which will allow one to study the role of normal genetic variation in phenotypic differences within a species (Drosophila Board Reference Panel White Paper 2008; www.flybase.org).
Such deep sequencing within a species, such as in D. melanogaster, will allow investigators to identify “quantitative trait nucleotides” that are responsible for a quantitative trait, which is one of the goals of “personalized medicine” (Ruden, 2007). Personalized medicine is one of the primary goals of pharmacology, in which one goes from an individual’s genetic makeup and disease phenotype to an optimal drug and dose that is customized for the individual. Having an individual’s complete genomic sequence, which will soon be provided as a standard laboratory protocol, will potentially allow one to determine the individual differences in response to drugs and susceptibility to disease. Having multiple whole-genome sequences allows the identification of quantitative trait nucleotides (QTN), which are the sequence polymorphism(s) that are responsible for a phenotype (De Luca et al., 2003).
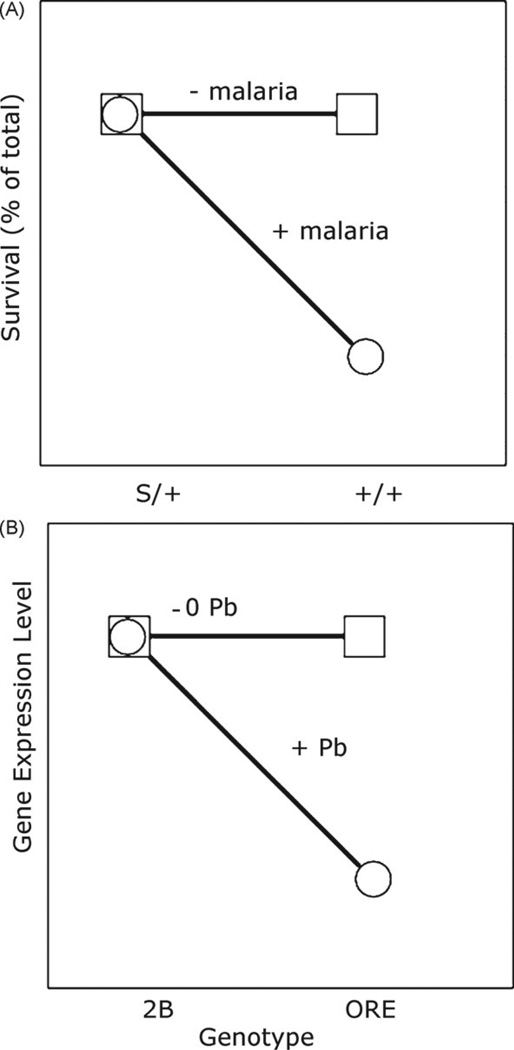
In addition to individual variation in the response to drugs, people also differ in sensitivity to other environmental insults such as infections or toxins. This phenomenon is known as a genotype-by-environment (G × E) interaction (Mackay and Anholt, 2007). The best-known G × E interaction is the sickle-cell-anemia allele of hemoglobin providing resistance to the malarial parasite Plasmodium falciparum (Fig. 1A). The general characteristic of G × E graphs, which are also know as “reaction norm graphs,” is that there is significant “crossing of the lines” as determined by analysis of variance (ANOVA) (Mackay and Anholt, 2007). We have investigated G × E interactions on a genomic scale, characterizing over 18,000 traits at once by investigating natural variation in fruit fly gene expression. Global gene expression levels were measured in fruit flies raised in control and lead-exposed environments. We have identified several thousand genes that have significant G × E interactions in which the genotypes are ORE and 2B and the environments are plus or minus lead (Fig. 1B). Both genes have similar expression levels in the absence of lead. In the presence of lead ORE expression is decreased but 2B is not. This technique is called “genetical genomics” (Jansen and Nap, 2001) and we call our neurotoxicology variation of this approach “genetical toxicoge-nomics” (Page and Ruden, 2005). We show that multiple regulators of several neurodevelopmental genes have evolved sensitivity to lead. We believe that our study has major implications in the role of natural variation in nervous system development in a toxic environment.
Fig. 1.
Genotype-by-environment interactions. (A) Heterozygosity for the sickle cell anemia allele of hemoglobin (S/+) confers resistance to malaria. Homozygosity for wild-type hemoglobin (+/+) confers susceptibility to infection by Plasmodium, and this leads to increased mortality. (B) Drosophila genes with GxE-eQTL have similar expression levels in the absence of lead (−Pb), but have a decrease in expression in the presence of lead (+Pb) in one genotype but not the other. Shown is repression by lead when the genotype is ORE but not 2B.
By assessing differential behavioral responses to developmental lead exposure among RILs we identified a G × E interaction for a behavioral trait (locomotor activity of adult flies) affected by lead. We observed a significant statistical association between the effect of lead on average daytime locomotor activity across lines and one marker locus, 30AB, on chromosome 2; we defined this as a Quantitative Trait Locus (QTL) associated with behavioral effects of developmental lead exposure. When 30AB was from 2B, lead significantly increased locomotor activity, whereas, when 30AB was from ORE, lead decreased it. 30AB contains about 125 genes among which are likely “candidate genes” for the observed lead-dependent behavioral changes. To the best of our knowledge, this is the first identification of a portion of the D. melanogaster genome that is involved in a behavioral response to lead (Hirsch et al., 2009). Using D. melanogaster, we have identified a “critical period” (the first 4 days of adult life) during which lead affects adult behaviors. We are thus able, in this species, to study behavioral (Hirsch et al., 2003), synaptic (Morley et al., 2003) and genetic changes resulting from chronic lead exposure during development.
Drosophila as a model for ethanol-induced behavior and neurotoxicity
Ulrike Heberlein1,2, 3, Ammon B. Corl1, Karen Berger3, Selena Bartlett3, and Rachael French2
Program in Neuroscience1, Department of Anatomy2, University of California, San Francisco, CA, and Ernest Gallo Research Center3, Emeryville, CA, USA
The Epidermal Growth Factor (EGF) receptor pathway regulates ethanol-induced behaviors in Drosophila and rodents. Alcohol is one of the most highly consumed and abused drugs in the world. Its pleasurable effects have been enjoyed by humankind for thousands of years. For some, however, alcohol consumption leads to addiction, a devastating illness with enormous medical and societal costs. In the United States, approximately 7–10% of adults suffer from alcohol use disorders (AUDs), costing the country over $200 billion per year and leading to over 75,000 deaths (Diamond and Gordon, 1997; Volpicelli, 2001). A better understanding of the genetic and environmental factors that contribute to the development of AUDs would therefore provide considerable benefits to those who suffer its direct consequences and to society in general.
The fruit fly, Drosophila melanogaster (D. melanogaster), has proven to be a useful model systemin which to study the genes and pathways that mediate acute and chronic behavioral responses to ethanol (Guarnieri and Heberlein, 2003; Wolf and Heberlein, 2003). Upon acute ethanolexposure,flies exhibit behaviors similar to those observed in mammals: low ethanol doses result in hyperactivity, whereas higher doses cause decreased activity and eventual loss of postural control and sedation(Singh and Heberlein, 2000; Wolf et al., 2002). Unbiased genetic approaches and candidate gene analyses have provided insight into various molecules and biochemical pathways that regulate the ethanol response in Drosophila (Moore et al., 1998; Park et al., 2000; Corl et al., 2005; Wen et al., 2005; Rothenfluh et al., 2006) as well as the responsible neuroanatomical loci (Rodan et al., 2002; Urizar et al., 2007). Several of the molecules implicated in ethanol-related behaviors in Drosophila, such as protein kinase A (PKA), calcium-dependent adenylate cyclase (Moore et al., 1998) and the fly orthologue of neuropeptide Y, NPF (Wen et al., 2005), have been shown to have similar roles in mammals (Thiele et al., 2000, 2002; Maas et al., 2005), validating Drosophila as a valuable tool for identifying molecular pathways underlying the behavioral response to ethanol.
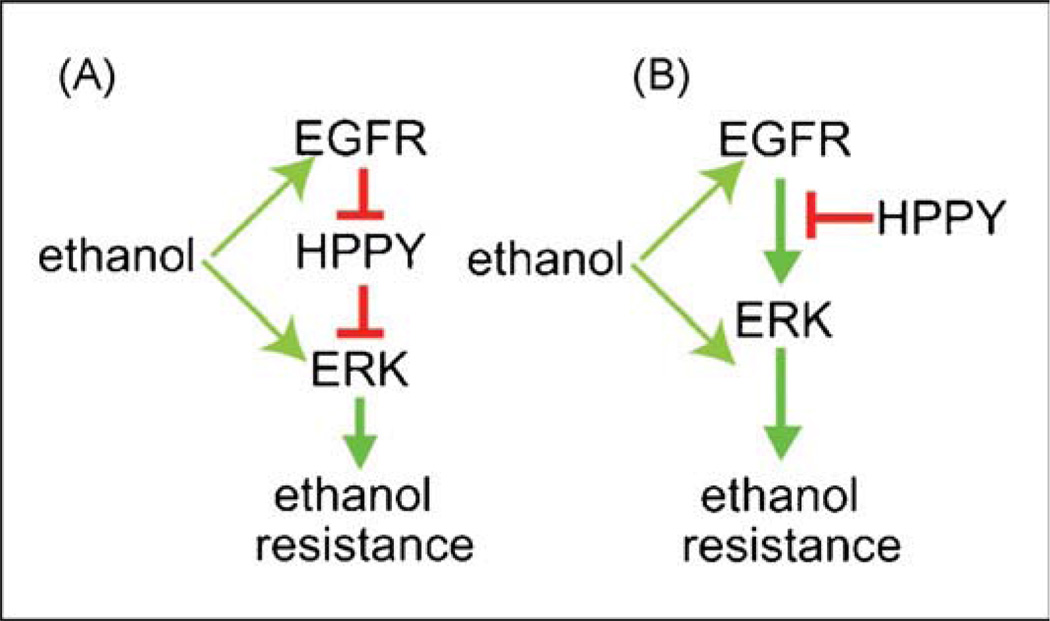
To expand our knowledge of the molecular components underlying the behavioral effects of ethanol, we conducted a genetic screen in Drosophila and identified a mutant, happyhour (hppy), due to its resistance to the sedative effects of ethanol. Although the Hppy protein shows strong homology to mammalian Ste20-family kinases involved in Jun N-terminal kinase signaling, genetic manipulations of this pathway did not alter ethanol sedation. However, perturbations of the Epidermal Growth Factor receptor (EGFR) pathway in specific neuronal populations, particularly dopaminergic neurons and insulin-producing cells, strongly affected sensitivity to ethanol-induced sedation. Genetic and biochemical experiments suggest that Hppy functions as an inhibitor of the EGFR pathway (Fig. 2). Finally, acute pharmacological inhibition of the EGFR in adult animals caused altered acute ethanol sensitivity both flies and mice, and in reduced ethanol consumption in a preclinical rat model of alcoholism. Inhibitors of the EGFR or components of its signaling pathway are thus potential pharmacotherapies for alcoholism (Corl et al., 2009).
Fig. 2.
Models for EGFR- and HPPY-regulation of ethanol sensitivity. The EGFR pathway promotes resistance to ethanol-induced sedation and HPPY functions to counteract this effect by inhibiting the EGFR pathway. Ethanol may act on the EGFR directlyorondownstream signaling molecules, including ERK. (A) Amodelinwhich HPPY is directly inhibitedby EGFR activation, (B) amodelinwhich HPPY function as a constitutive inhibitor of the pathway. Red intersecting lines represent inhibitory interactions, green arrows represent activating interactions. EGFR = Epidermal Growth Factor Receptor, ERK = Extracellular signal-Regulated Protein Kinase, HPPY = Happyhour protein (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.).
A Drosophila model for ethanol-induced neurotoxicity. It has long been known that heavy alcohol consumption leads to neuronal death and neurobehavioral pathology (Martin et al., 1986; Bengochea and Gonzalo, 1990; Phillips, 1990; Arendt, 1994; Agartz et al., 1999; Obernier et al., 2002a,b; Rupp et al., 2003), Approximately 10% of alcoholics develop alcoholic dementia, Wernicke–Korsakoff’s syndrome and other cognitive disorders (Eckardt and Martin, 1986; Martin et al., 1986; Fadda and Rossetti, 1998). Alcoholic brains display atrophy in many areas, including the frontal lobes, diencephalon, basal forebrain, hippocampus, cerebellum, and basal ganglia (Martin et al., 1986; Bengochea and Gonzalo, 1990; Fadda and Rossetti, 1998; Agartz et al., 1999). In humans and rats, chronic alcohol exposure leads to cholinergic neuron loss in the basal forebrain, leading to memory impairment (Arendt, 1994; Fadda and Rossetti, 1998). In addition, both alcoholic humans and rodent alcoholism models show olfactory damage; in rodent models, two days of acute ethanol exposure causes the death of olfactory neurons, followed by retrograde degradation in the temporal dentate gyrus and regions of the hippocampus known to be involved in olfaction and memory (Switzer et al., 1982; Collins et al., 1996; Obernier et al., 2002b), while in one study, more than half of alcohol-dependent humans could be classified as hyposmic (Rupp et al., 2003).
Multiple hypotheses have been presented to explain ethanol-induced brain damage. The mechanisms proposed vary depending on cell type and developmental stage (Fadda and Rossetti, 1998). For example, alcoholism frequently leads to thiamine deficiency. When thiamine deficiency is induced experimentally in rodent models, it leads to neurological damage similar in some respects to that seen in chronic alcoholics (Fadda and Rossetti, 1998; Gibson and Zhang, 2002). Nevertheless, thiamine deficiency does not mimic all types of damage induced by chronic alcohol use, such as hippocampal abnormalities, shrinkage of the cerebral cortex, and cholinergic loss in the basal forebrain. Other mechanisms proposed to explain these effects are production of reactive oxygen species and increased production of polyamines, both of which are known to occur in response to alcohol exposure (Fadda and Rossetti, 1998; Sun et al., 2001). Finally, ethanol is known to bind to N-methyl-D-aspartate (NMDA) receptors, and it is thought that this interaction explains many of the drug’s neurotoxic effects (Weight et al., 1993; Faingold et al., 1998; Wirkner et al., 1999; Woodward, 1999). Chronic ethanol exposure inhibits NMDA receptors, leading to a compensatory increase in glutamatergic neurotransmission (Lancaster, 1992; Fadda and Rossetti, 1998). These neurons are thus hyperexcitable upon ethanol withdrawal, and this leads to excessive intracellular Ca2+, mitochondrial damage, and, ultimately, activation of apoptotic pathways (Fadda and Rossetti, 1998). In addition, acute ethanol-induced death in cortical neurons is enhanced by treatment with the NMDA receptor agonist MK-801 (Corso et al., 1998), indicating that, in some cell types, ethanol directly exerts an acute excitotoxic effect.
Glycogen synthase kinase 3β (GSK-3β) was first identified for its ability to phosphorylate glycogen synthase (Embi et al., 1980), and has since been shown to have many functions in development and behavior, including both inhibition and activation of cell death pathways (Beurel and Jope, 2006). In models of neurode-generative disease, GSK-3β is required for both β-amyloid-associated neurotoxicity (Takashima et al., 1993; Alvarez et al., 1999) and tau-mediated neurodegeneration (Lucas et al., 2001; Jackson et al., 2002). In addition, GSK-3β can mediate excitotoxic cell death (Facci et al., 2003; Takadera et al., 2004) as well as ethanol-induced apoptosis of cultured neurons (Takadera and Ohyashiki, 2004).
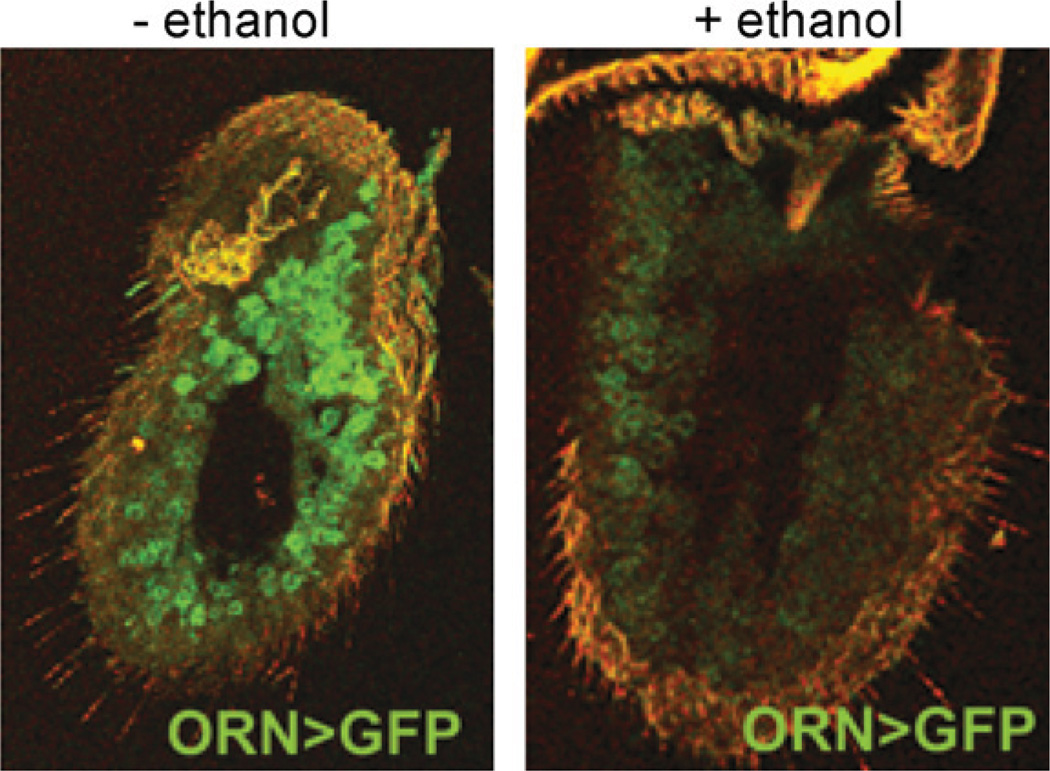
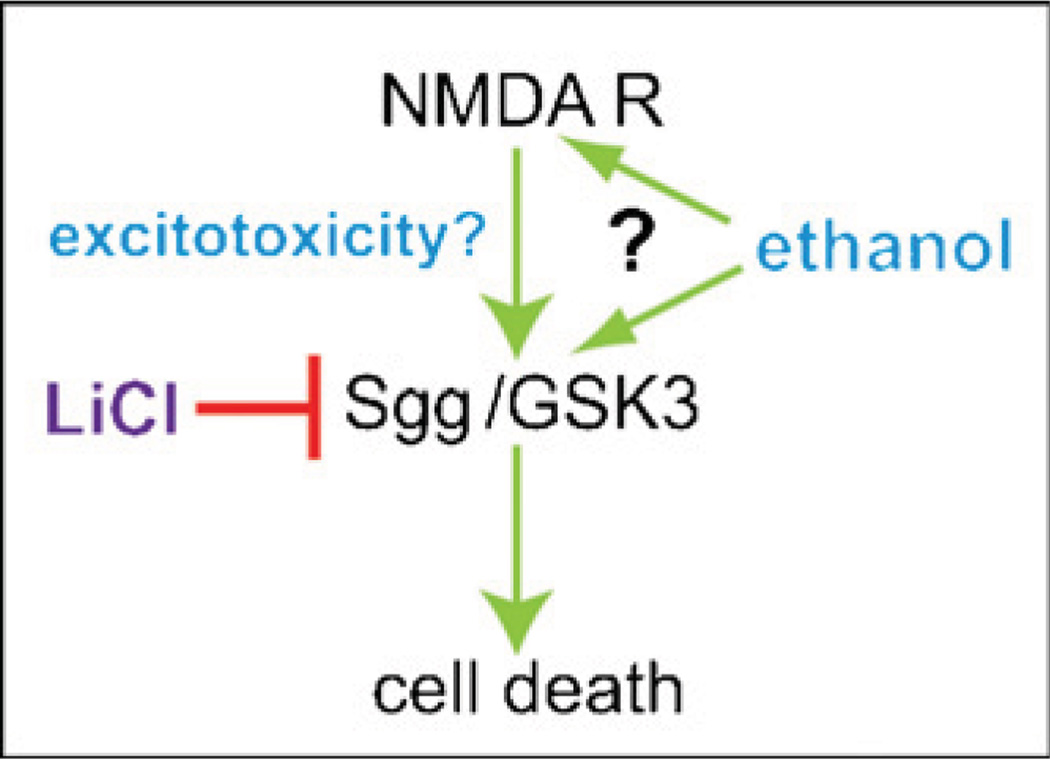
In flies, we have shown that exposure to even a single sedating dose of ethanol leads to widespread cell death in the antennae, the primary olfactory organs of flies (Fig. 3). Ethanol-induced death of Drosophila olfactory neurons is apoptotic in nature, requires shaggy (sgg), the Drosophila homolog of GSK-3β, and can be prevented by treatment with the GSK-3β inhibitor LiCl. In addition, we show that the neurotoxic effects of ethanol exposure can be blocked by electrical silencing of the olfactory neurons, demonstrating that ethanol-induced death in these cells is due to excitotoxicity. Finally, we demonstrate that ethanol-induced neuronal death requires NMDA receptors in the olfactory neurons, and that sgg and the NMDA receptor are likely acting in concert to mediate this effect (French and Heberlein, in revision) (Fig. 4).
Fig. 3.
Ethanol exposure causes death or olfactory receptor neurons. Confocal images of third antennal segments are shown. Olfactory receptor neurons (ORNs) are labeled with greenfluorescent protein (GFP). Asingle sedating doseof ethanol is sufficient to induce the loss of ORNs (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.).
Fig. 4.
Model for ethanol-induced cell death. Ethanol, possibly acting through NMDA receptors (NMDAR) activates GSK3 (Shaggy/Sgg in Drosophila) to promote cell death. Lithium chloride inhibits GSK3 thus preventing ethanol-induced death.
In vivo studies of the effects of acute ethanol exposure on the adult nervous system have been rare, and while previous studies have shown links between ethanol exposure and activity-dependent neuron death, or between GSK-3β and excitotoxicity (Acquaah-Mensah et al., 2002; Ikonomidou et al., 2000; Olney et al., 2000, 2002), to our knowledge, ours is the first work to link activity-dependent or excitotoxic death, ethanol-induced neuronal apoptosis, and GSK-3β function in a single, adult, in vivo system. Finally, while the incidence and severity of alcoholic neurodegeneration is clearly affected by genetic background (Martin et al., 1986; Becker et al., 1996; Gilliam et al., 1997; Olney et al., 2002; Crews and Braun, 2003; Crews et al., 2004; Goodlett et al., 2005; Warren and Li, 2005), there has been limited success identifying the genes involved, due to the limited range of genetic techniques available in most vertebrate models. By comparison, Drosophila is amenable to unbiased, rapid, and complex genetic manipulations. Thus, we hope to use our model for ethanol-induced neuronal cell death to identify genes and mutations involved in sensitivity to ethanol neurotoxicity. In addition, our model will allow greater understanding of the molecular processes of neuronal death in alcoholic dementia.
Zebrafish and mouse models and genetic approaches to behavioral neurotoxicology
Edward D. Levin, Donnie Eddins and Elwood Linney
Department of Psychiatry & Behavioral Sciences and Department of Microbiology & Genetics, Duke University Medical Center, Durham, NC, USA
A hierarchy of animal models is necessary to accurately determine the neural and molecular mechanisms of toxicity underlying behavioral dysfunction. These can be used in a complementary fashion. Vertebrate animal models are needed in addition to the study of invertebrate species to provide complex systems more closely resembling human neurobiology. Piscine models including zebrafish have proven to be valuable because of their continuous visual access during early development due to their clear chorion and because of the wide variety of mutants available for study and the availability of morpholinos, which allow specific aspects of genetic suppression during early development. Mouse models have long been used to understand genetic aspects of neurobehavioral function. The availability of various gene knockouts provides models with which to study the roles of those factors for neurobehavioral function and effects of toxicants on those functions. Both zebrafish and mouse models have been quite valuable in our studies of the developmental neurotoxic effects on later behavioral function.
Zebrafish models of the behavioral toxicity of developmental chlorpyrifos. Zebrafish have recently become widely used for determining molecular mechanisms for neurotoxic effects on behavior. Zebrafish have a well-established literature concerning the molecular bases of development in general and neurodevelopment in particular. There are a wide variety of mutant zebrafish lines available which facilitate the discovery of critical molecular mechanisms of nervous system development. The morpholino technique in zebrafish provides a way to reversibly suppress a genetic factor during early development and is particularly useful in helping determine the critical molecular targets for developmental neurotoxicants. Recently, advances have been made in the behavioral assessment of zebrafish, particularly concerning neurobehavioral plasticity from elementary sensorimotor habituation and locomotor adaptation through more complex associative learning and memory processes. Computerized video tracking systems have been quite helpful in this effort. For example, we have found that a morpholino preparation in zebrafish which specifically inhibits acetylcholinesterase (AChE) is very useful for determining the degree to which AChE inhibition vs. other mechanisms are responsible for the persisting behavioral neurotoxicity of developmental chlorpyrifos exposure (unpublished data).
Zebrafish are becoming widely used to study neurodevelopmental defects associated with toxicant exposure (Scholz et al., 2008) as well as neurological diseases (Best and Alderton, 2008). Zebrafish have many neurobehavioral systems in common with mammals. Like rats and mice, proper cholinergic functioning in developing zebrafish is key for the normal development of the nervous system (Best and Alderton, 2008). Other transmitter systems such as dopamine, norepinepherine and serotonin are also represented in zebrafish (Drapeau et al., 2002; Kaslin and Panula, 2001; McLean and Fetcho, 2004; Wullimann and Mueller, 2004). Zebrafish have extensive cognitive abilities and can learn spatial and color discrimination (Arthur and Levin, 2001; Colwill et al., 2005; Levin and Cerutti, 2008; Levin and Chen, 2004; Levin et al., 2003, 2006a; Williams et al., 2002). Zebrafish models of the neurobehavioral effects of development can be important complementary models for understanding neurotoxicity. Together with classic rodent and primate models as well as other non-mammalian models, zebrafish could help elucidate the mechanistic bases for neurotoxicant-induced behavioral impairment.
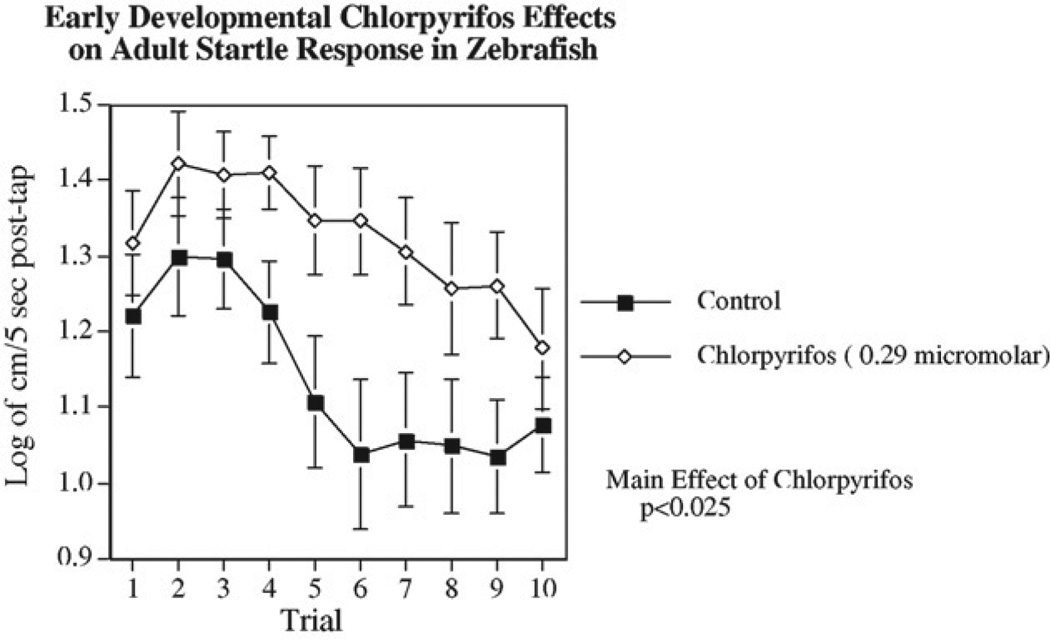
We have developed test methods for indexing behavioral function in adult zebrafish. Spatial and color discrimination learning can be effectively assessed in a three-chamber choice task (Arthur and Levin, 2001; Levin and Chen, 2004; Levin et al., 2003, 2006a). The three-chamber task is particularly effective for differentiating response latency from choice accuracy since the same response (albeit in a different direction) is required for a correct as for an incorrect choice. With the three-chamber task we have shown that adult zebrafish developmentally exposed to chlorpyrifos (CPF) at tank water concentrations of 10 or 100 ng/ml on days 0–5 post-fertilization, had persisting defects in both delayed spatial alternation accuracy and latency of response (Levin et al., 2003). This study was important in showing the persisting neurobehavioral effects after early developmental exposure, however, the delayed spatial alternation task takes considerable periods of training. To increase the rate of testing we developed an automated assessment of startle response and its habituation. Startle response was chosen because it provides a quick measure of both sensory and motor integration and it shows a rapid habituation curve with repeated trials, which gives information concerning neuroplasticity. Startle response and habituation in rodents is very well characterized, neural mechanisms are known and it is a sensitive indicator of pharmacological and toxicological treatments (Karl et al., 2003; Swerdlow et al., 2001). We have found that postnatal exposure of rats to parathion an organophosphate (OP) pesticide significantly decreased startle response (Timofeeva et al., 2008). In zebrafish, both startle response and its habituation have been studied in embryos (Best et al., 2008; Burgess and Granato, 2007; Weber, 2006). Developmental mercury exposure was shown to increase startle reactivity (Weber, 2006). We developed a startle response test for adult zebrafish to determine the persisting effects of developmental toxicant exposure. We found that ten trials with a 1-min interval provided a rapid but reliable measure of startle response and its habituation. The hypothesis of this study was that the automated characterization of startle response in zebrafish would provide a rapid and sensitive indicator of persisting neurobehavioral impairment caused by early developmental exposure to chlorpyrifos at a dose that previously had been shown to cause a learning impairment (Levin et al., 2003). The benchmark 0.29 mM dose (100 ng/ml) of CPF was chosen because it had been previously shown to cause marked impairment in memory function without causing increases in mortality or obvious morphological malformations. As shown in Fig. 5, this CPF dose caused significant increase in startle response (Eddins et al., 2009). The main effects of CPF exposure and trial number on startle response without a significant interaction between the two factors, though there did appear to be a suggestion of slower habituation in the CPF-exposed group. CPF inhibits acetylcholinesterase, which provides indirect agonist effects at nicotinic and muscarinic acetylcholine receptors. Thus, we tested the persisting impacts of developmental exposure to direct agonists of these receptors nicotine and pilocarpine. Effects of developmental exposure to these drugs were tested at doses subthreshold for causing morphologic malformation and lethality in the embryos. Both nicotine and pilocarpine caused persisting increased startle response but at much higher concentrations than CPF (Eddins et al., 2009). In a rodent study we found another OP parathion to significantly decrease startle response (Timofeeva et al., 2008), but this was a consequence of briefer exposure during the postnatal period rather than the continuous exposure to CPF from conception through the first 5 days of development in zebrafish. Finally, the effects of developmental CPF exposure on the monoaminergic neurotransmitters dopamine, norepinepherine and serotonin were tested both immediately after exposure (day 6) and long after exposure (approximately 20 weeks) since monoaminergic systems have been shown to be affected by developmental CPF exposure in rats (Aldridge et al., 2005, 2003; Johnson et al., 1998; Slotkin and Seidler, 2005, 2007; Slotkin et al., 2002). This neurochemical analysis was conducted to determine the possible involvement of these transmitters in the neurobehavioral impairments. In adult zebrafish after developmental CPF exposure brain dopamine but not serotonin or norepinepherine was significantly decreased. In young fish just after the end of exposure all three transmitters dopamine, serotonin and norepinepherine were significantly lowered.
Fig. 5.
Zebrafish exposed to chlorpyrifos during development and startle response. Exposure of zebrafish during the first 5 days post-fertilization significantly (p < 0.025) increased swimming activity after a repeated tactile startling stimulus (Eddins et al., 2009).
Methallothionein knockout mice and cognitive effects of mercury. Mice are classic models for determining gene × environment interactions in general as well as with behavioral neurotoxicity in particular. Knockout mice can be especially useful for determining critical molecular mechanisms for neurobehavioral toxicity. If an essential mechanism for a toxicant is knocked out the neurobehavioral effect should be eliminated. Critical targets for some classes of toxicants such as metals can be complex. Not only multiple targets, but also distributional mechanisms must be considered in the gene × environment interactions. Metallothioneins (MT) are a group of small proteins which bind a variety of transition metals including mercury, primarily in the inorganic form. We have found that mice with knockouts of MT types 1 and 2 are more susceptible to the early learning deficits caused by developmental mercury compared to wild-type control mice (Eddins et al., 2009).
Metallothioneins are known to be key in the physiology of transition metals, but their roles in neurobehavioral function have not yet been well characterized. Alterations in metallothionein function could alter neural function in ways that would impair behavioral function. For example, the hippocampus, which plays a central role in cognitive function depends on the actions of zinc in the hippocampal mossy fiber system (Daumas et al., 2004). Metallothioneins 1 and 2 protect against the adverse effects of both zinc underload and zinc overload (Kelly et al., 1996). MT 1 appears to play a greater role in metal metabolism whereas MT 2 appears to play a greater role in cell growth (Kobayashi and Sayato-Suzuki, 1988). In the striatum, alterations of copper functions can lead to cognitive and behavioral impairments seen in Wilson’s disease (Portala et al., 2001). Other transition metals such as mercury have no identified normal physiological role, but are environmental contaminants, which have known neurotoxic actions, which result in behavioral abnormalities including cognitive impairment (Goulet et al., 2003; Peixoto et al., 2007; Yoshida et al., 2005). Abnormalities in metallothionein function could alter neurobehavioral function through dysregulation of these transition metals.
To begin determining the potential roles metallothioneins may play in neurobehavioral function we have tested the effects of knocking out metallothionein 1 and 2 genes on cognitive function. The impact of metallothionein 1 and 2 knockout on choice accuracy and locomotor behavior in the 8-arm radial-arm maze was determined during acquisition and maintenance of response, with drug treatment and after toxicant challenge.
In our initial set of studies we found that mice with null mutations for metallothionein 1 and 2 from Jackson Labs showed a significant impairment in choice accuracy on the 8-arm radial maze test of spatial learning and memory (Levin et al., 2006b). This was seen both in younger adult and older adult mice (Levin et al., 2006b). Interestingly, the impairment of the metallothionein knockout mice vs. their wild-type controls (129 strain) was significantly ameliorated by acute nicotine treatment (Levin et al., 2006b). This indicated that the metallothionein knockout-induced impairment was not due to learning per se because the effect of nicotine was immediate and did not depend on further training in the maze. More likely to attentional or memory aspects of the task which could be immediately changed from session to session as the nicotine treatments changed.
In a recent study we have shown that metallothionein knockout mice show a greater adverse effect of neonatal mercury (HgCl2) exposure (Eddins et al., 2008). In that study, a significant impairment in choice accuracy on the radial-arm maze was seen in metallothionein knockout mice with a history of neonatal mercury exposure during the initial phase of training. During this initial phase neither mercury alone nor the metallothionein knockout alone caused impairment but the combination did.
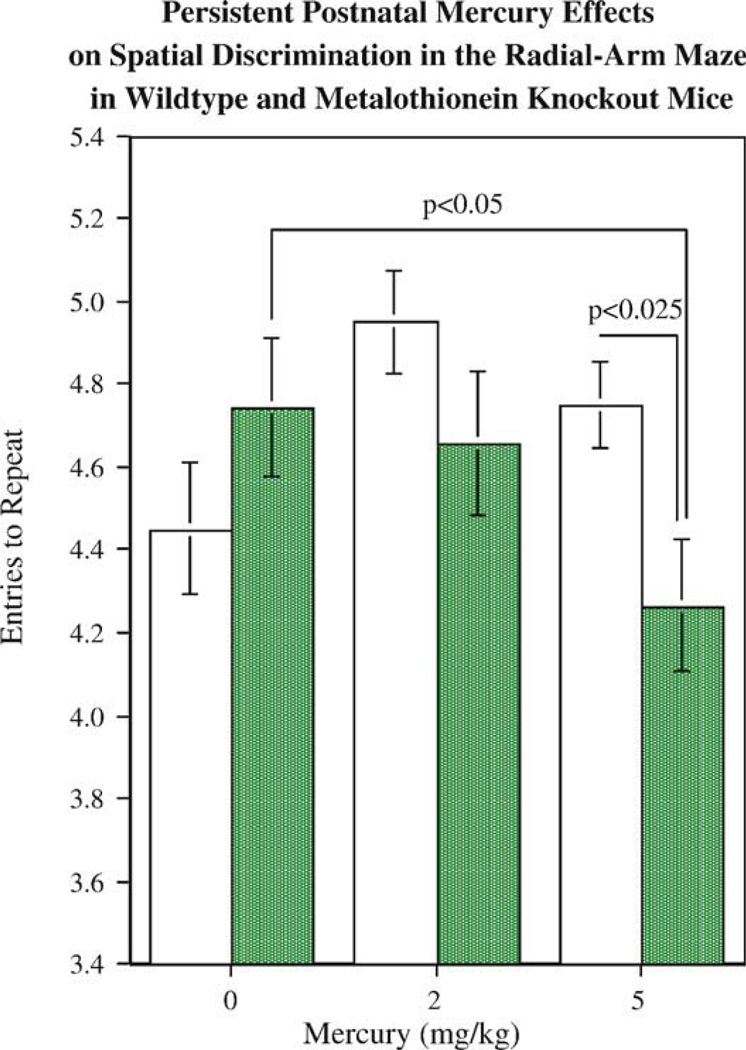
Developmental exposure to metals such as mercury has long been known to impair cognitive function. The mechanisms controlling metal distribution such as the metallothioneins can influence actions of neurotoxic metals as well as essential metals. To characterize the role of metallothionein in learning and memory, mice with null mutations (knockout) of genes for metallothionein 1 and 2 were trained on a win-shift task in the 8-arm radial maze. Metallothionein 1–2 knockout mice had a similar choice accuracy levels as wild-type controls in the early stage of training, but had impaired choice accuracy during the later stages of training (Levin et al., 2006b). To determine the influence of metallothionein on developmental mercury neuro-behavioral toxicity, mercuric chloride was injected (0, 2 and 5 mg/kg, sc) on days 7, 14 and 21 after birth (Eddins et al., 2008). As shown in Fig. 6, during the early stage of training (sessions 1–6) the metallothionein knockout l mice exposed to 5 mg/kg mercuric chloride had significantly lower accuracy scores than either wild-type mice exposed to the same mercury dose or metallothionein knockout mice not exposed to mercury. The entries to repeat measure of choice accuracy indexed the number of consecutive correct arm entries before the first repeated arm entry (error). This takes into account the fact that this radial-arm maze task becomes more difficult as session progresses and there are fewer correct choices remaining. During the later part of the training sequence the effect of selective mercury-induced impairment in the methallothionein knockout animals gave way to a more general metallothionein knockout-induced impairment in both mercury treated and unexposed animals. In addition there were persistent neurochemical effects. Dopamine (DA) in the frontal cortex was differentially affected by developmental mercury exposure in metallothionein knockout and wild-type mice. The 5 mg/kg dose of mercury caused a significant increase in frontal DA levels in the wild-type mice. This effect was not seen in the metallothionein knockout mice. The increase in frontal DA may have been key in preventing functional impairment by mercury in the wildtype mice by increasing the role of the frontal cortex in solving the task. The lack of this response in the MT knockout mice may have limited their compensation for mercury-induced damage. Metallothionein knockout significantly increased frontal cortical levels of serotonin and its metabolite 5-HIAA regardless of mercury condition. Metallothionein knockout mice were more sensitive to the persisting adverse cognitive effects of developmental mercury exposure. Alterations in frontal cortical DA response may be related to the functional impairment.
Fig. 6.
Mice with knockouts of metallothionein exposed to mercury during development. Metallothionein 1 and 2 knockout mice showed significantly (p< 0.05) greater radial-arm maze choice accuracy impairment resulting from postnatal mercury (HgCl) exposure (Eddins et al., 2008). The choice accuracy measure is entries to repeat, the number of consecutive correct arm entries before the first repeat error.
Metallothioneins play important roles in neural systems relating to cognitive function. Significant impairments of radial-arm maze choice accuracy are seen with knockouts of metallothioneins 1 and 2. This accompanied by substantial increases in frontal cortical serotonin, which may be related to the cognitive impairment. The acute reversibility of the methallothionein knockout-induced choice accuracy deficit with nicotine indicates that the cognitive deficit was not one of impaired learning but more likely involved memory or attentional impairment. Metallothionein 1 and 2 knockout made mice more sensitive to the lasting adverse effects of mercury on radial-arm maze acquisition. This increased sensitivity to mercury effects may be related to a diminished response of dopamine systems in the frontal cortex.
Zebrafish and mice along with a spectrum of other species are valuable for determining gene × environment interactions in the basis of neurobehavioral toxicity. The use of morpholino and knockout techniques to assess the impact of genes of interest have provided important mechanistic information. Further advances in molecular analytic techniques will permit broad screens of the contribution of many polymorphisms to increase or decrease susceptibility to neurobehavioral damage after developmental toxicant exposure.
Prevention of Alzheimer’s disease and cognitive decline: the role of genes and environment
Kathleen A. Welsh-Bohmer and Kathleen M. Hayden, for the Cache County Study Investigators
Department of Psychiatry and Behavioral Sciences, Joseph and Kathleen Bryan Alzheimer’s Disease Research Center, Duke University, Durham, NC 27710, United States
Alzheimer’s disease (AD) is a recognized global public health problem, cutting across cultural, economic and demographic lines (Brookmeyer et al., 2007). It is estimated that 24 million people are currently affected worldwide with dementia. This is expected to climb to 42.3 million in 2020 and 81.1 million in 2040. The etiology of AD and the factors that influence the onset of disease symptoms are not yet resolved, but likely involve a complex interaction between genetic predisposing factors and environmental exposures acquired across the lifespan, from early development through midlife and later old age. The epidemiological work we are conducting in an exceptionally long-lived population in Utah, the Cache County Memory Study (CCMS), examines both the genetic and environmental contributions to AD risk and their effects on the expression of the disease. We summarize here recent findings suggesting a role of the environment in modifying risk, symptom onset, and disease expression over time.
Genetic factors. By far the most influential risk factors for AD are inherited disease susceptibility factors. Three causative genes for AD related to amyloid processing have been identified (PS1, PS2, and APP), but together they explain only a small percentage (5–10%) of AD cases. Beyond these genes, a number of candidate “susceptibility” genes have been suggested that may act to modify risk and disease expression (Mayeux, 2003; Pericak-Vance et al., 2000; Perls, 2002). Apolipoprotein E (APOE) has been now firmly established as a major AD susceptibility gene (Strittmatter et al., 1993). Found in nearly half of all patients with AD, presence of an APOE ε4 allele confers an overall enhanced risk of AD dementia at the population level, as well as an earlier age of disease onset (Corder et al., 1993). However, unlike the known causative genes for AD, possession of the ε4 genotype does not confer absolute risk of developing AD. Substantial proportions of elderly even with ε4/ ε4 and ε3/ε4 genotypes may not develop AD in late old age (Khachaturian et al., 2004; Saunders et al., 1993). Other disease susceptibility genes and perhaps genes that confer cognitive robustness into late life, have been suggested by centenarian studies (Perls, 2002), family history investigations of dementia (Kaye, 1997; Mayeux, 2006) and recent genome wide association scans (Bertram et al., 2007). However, few of the associations and SNPs reported, with the exception of the recent report of variants in the SORL1 neuronal sorting receptor (Lee et al., 2007; Rogaeva et al., 2007), have yielded consistent findings, likely due in part to small effect sizes.
Environmental, gender and temporal modifiers. It is clear that genes are not solely responsible for AD risk and symptom onset (Gatz et al., 1997; Plassman et al., 2006). A number of environmental factors appear to exert substantial effects on the population’s variation in predisposition to AD (Breteler et al., 1991). Some of the factors implicated are not considered modifiable (e.g. advancing age and female gender). Others are considered treatable or avoidable, such as head injury (Plassman et al., 2000), thyroid disease (Heyman et al., 1984), and health conditions, including stroke and vascular risk conditions (e.g. diabetes, hypertension) (Esiri et al., 1999; Hofman et al., 1997; Skoog, 1998). A number of more tentative links have been made between common pharmacological exposures and lifestyle variables that may delay onset of dementia, presumably by affecting the trajectory of cognitive decline (Dosunmu et al., 2007; Larson et al., 2006). Included here are medications (Fotuhi et al., 2008; Zandi et al., 2002, 2004, 2005), cognitive engagement (Fratiglioni et al., 2004) and education (Gatz et al., 2001) across the lifespan, and some non-pharmacological lifestyle habits (e.g. diet and exercise) (Cotman and Berchtold, 2002; Scarmeas et al., 2001, 2006; Wengreen et al., 2007). These potentially modifiable factors have appeal as they suggest possible strategies for disease prevention.
Environmental toxicants. Among the more speculative risk factors for AD are environmental toxicants, such as organic solvents, air-borne pollutants, and heavy metals. These have been linked to a number of neurological disorders including Parkinson’s disease and multiple sclerosis (Baldi et al., 2003; Kamel et al., 2007). A role for these compounds in AD has been suggested, but very few studies have been conducted and the risk relationships of these exposures with AD are not well established (Santibanez etal., 2007). If related to AD risk, they have implications for understanding the pathogenesis of the disease and may point to treatment strategies.
In the CCMS study of AD and related dementias (5092 participants, aged 65+), we have examined a number of common environmental toxicants and their influence on overall AD risk, onset of dementia, and cognitive decline in old age. Among the toxic exposures we have examined are pesticides and herbicides. At the baseline visit in 1995, detailed occupational exposure questionnaires were collected which tabulated information about exposures to these compounds and the types used. With the benefit of over 10 years of observation and two additional waves of diagnostic assessment (3 and 8 years after baseline), we have been able to explore the relationship between prior pesticide exposure and the risk of incident dementia and AD. Preliminary analyses have been conducted in the 4012 individuals without dementia who at the baseline visit provided complete information on occupational exposures. In this population of older adults, significantly more men reported exposures than women (p < 0.0001). Because so few women reported the exposures of interest, we restricted the analysesto the men in the cohort sample (n = 1929). No significant age or education differences were observed between the exposed (n = 682) and unexposed groups (n = 1247).
At follow-up 8 years later, 78 individuals had developed dementia. In logistic regression models adjusted for baseline age, education, and APOE genotype there was no observed risk for all cause dementia among individuals exposed to pesticides (Odds Ratio (OR): 0.97, 95%, Confidence Interval (CI) 0.73–1.29). However, when the outcome was restricted to AD, an increased risk was observed (OR: 1.99, 95% CI: 1.12–3.56). There was a trend towards an interaction between exposure to pesticides and APOE ε4 genotype (OR = 1.81 CI: 1.0–3.22), suggesting that the combination of genotype and exposure may influence the risk of incident AD. Further evaluation in larger samples may help clarify this association. Likewise, the effects of different classes of pesticides could not be examined meaningfully given the small number of individuals with exposures to particular classes and events. However, we note that organophosphates and organochlorines were the most commonly reported exposures in those that developed incident dementia.
The findings of from this initial analysis of the Cache data suggest an increased risk relationship between prior pesticide use and the later development of AD dementia (Hayden et al., 2009). The strength of the study is the extended observation period (8–10 years) which allows for more dementia events and hence the capability of examining relationships in a population with low exposure to the toxins of interest. Other similar population based studies with long intervals of follow-up, in France (Baldi et al., 2003) and Manitoba Canada (Tyas et al., 2001), report similar increased risk relationships between pesticides and AD. However, a case–control study from Quebec found no relationship after adjusting for education and APOE (Gauthier et al., 2001). Understanding the role of different classes of environmental toxins in AD onset and the interaction of these exposures with genetic predisposition will require much larger samples of exposed individuals, followed over extended periods of time, with good quantification of exposure. Work in large cohorts comprised of agricultural workers, as has been done in Parkinson’s disease (Kamel et al., 2007) would be ideal for furthering our understanding of the role of pesticide exposures in relation to AD. With advances in genetic analysis, investigation can proceed beyond candidate genes to include the role of individual single nucleotide polymorphisms and their interactions.
2. Conclusions
The ultimate goal of this symposium was to foster the development of a program that would encourage the use of powerful genetic and technological approaches and sophisticated behavioral assessments to determine the influence of environmental neurotoxicants in the gene-behavioral interrelationship. It is becoming obvious that good correspondence exists between the neurobehavioral toxicity seen in mammalian models and that seen in human epidemiologic studies. The recent advances in molecular biology have provided tools for understanding the roles of genetic expression in neurobehavioral toxicity. Genetic methods are being used in both human epidemiological studies as well as experimental mammalian models. Even greater progress can be made by incorporating complementary experimental models such as C. elegans, Drosophila and zebrafish. These more elementary models have considerable advantages for understanding the relationship between toxicant actions on genetic expression and neurobehavioral function. New molecular technologies will continue to emerge. The field of behavioral neurotoxicology benefits from the new molecular information garnered with transdisciplinary collaborations between behavioral scientists and molecular researchers providing new mechanistic information underlying behavioral toxicity. Behavioral scientists can play a key role in the development of sensitive, economical and reliable techniques to assess the behavioral function of the non-mammalian models. Especially important is the interchange between new models and traditional rodent models. As discussed at the symposium the new models offer the promise of high-throughput and detailed molecular information but they will not replace the traditional rodent models or human studies. Rather these new models can enhance the usefulness of these studies by discovering novel mechanisms of toxicity to be tested. Encouraging these types of interdisciplinary research is a priority in behavioral neurotoxicology.
Acknowledgements
Dr. Aschner’s research was supported in part by PHS grant NIEHS R01 ES10563.
Dr. Ruden’s research was supported by the Environmental Health Sciences Center in Molecular and Cellular Toxicology with Human Applications Grant P30 ES06639 at Wayne State University, NIH R01 grants (ES012933 and CA105349) to D.M.R.
Dr. Heberlein’s research was supported by NIH AA010035, The Wheeler Center for the Neurobiology of Addiction, the McKnight Foundation for Neuroscience and the Gallo Center of UCSF.
Dr. Levin’s research was supported by grants from the National Association for the Advancement of Autism Research, Autism Speaks and the Duke University Superfund Basic Research Center, ES010356.
Dr. Welsh-Bohmer’s research was supported by AG028377, AG011380, AG029336 and supplemental funds to AG11380 from NIEHS.
Footnotes
Conflict of interest
None.
References
- Acquaah-Mensah GK, Kehrer JP, Leslie SW. In utero ethanol suppresses cerebellar activator protein-1 and nuclear factor-kappa B transcriptional activation in a rat fetal alcohol syndrome model. J Pharmacol Exp Ther. 2002;301:277–283. doi: 10.1124/jpet.301.1.277. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. doi: 10.1016/s0014-5793(99)00685-7. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Anim Cognit. 2001;4:125–131. [Google Scholar]
- Arya U, Dwivedi H, Subramaniam JR. Reserpine ameliorates Abeta toxicity in the Alzheimer’s disease model in Caenorhabditis elegans . Exp Gerontol. 2009;44:462–466. doi: 10.1016/j.exger.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157:409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Randall CL. Teratogenic actions of ethanol in the mouse: a minireview. Pharmacol Biochem Behav. 1996;55:501–513. doi: 10.1016/s0091-3057(96)00255-9. [DOI] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol. 1990;5:349–357. [PubMed] [Google Scholar]
- Berkowitz LA, Hamamichi S, Knight AL, Harrington AJ, Caldwell GA, Caldwell KA. Application of a C. elegans dopamine neuron degeneration assay for the validation of potential Parkinson’s disease genes. J Vis Exp. 2008 doi: 10.3791/835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Best JD, Alderton WK. Zebrafish: an in vivo model for the study of neurological diseases. Neuropsychiatric Dis Treat. 2008;4:567–576. doi: 10.2147/ndt.s2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Cole RD, Anderson GL, Williams PL. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem. 2003;12:3049–3055. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, Freedman JH. Medium- and high-throughput screening of neurotoxicants using C. elegans . Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM, Jr, Klein JB, et al. Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta(1-42): implications for Alzheimer’s disease. Neurobiol Aging. 2006;27:1239–1249. doi: 10.1016/j.neurobiolaging.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neuro-degener Dis. 2004;1:175–183. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl. 2):S36–S42. doi: 10.1093/ije/20.supplement_2.s36. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister C, Luersen K, Heinick A, Hussein A, Domagalski M, Walter RD, et al. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) Faseb J. 2008;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Process. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corl AB, Rodan AR, Heberlein U. Insulin signaling in the nervous system regulates ethanol intoxication in Drosophila melanogaster . Nat Neurosci. 2005;8:18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione and MK-801. Alcohol Clin Exp Res. 1998;22:217–224. [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcohol Clin Exp Res. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res. 2004;28:350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans . Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Halley H, Lassalle JM. Disruption of hippocampal CA3 network: effects on episodic-like memory processing in C57BL/6J mice. Eur J Neurosci. 2004;20:597–600. doi: 10.1111/j.1460-9568.2004.03484.x. [DOI] [PubMed] [Google Scholar]
- De Luca M, Roshina NV, Geiger-Thornsberry GL, Lyman RF, Pasyukova EG, et al. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat Genet. 2003;34(4):429–433. doi: 10.1038/ng1218. [DOI] [PubMed] [Google Scholar]
- De Luca M, Yi N, Allison DB, Leips J, Ruden DM. Mapping quantitative trait loci affecting variation in Drosophila triacylglycerol storage. Obes Res. 2005;13:1–10. doi: 10.1038/oby.2005.196. [DOI] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dosunmu R, Wu J, Basha MR, Zawia NH. Environmental and dietary risk factors in Alzheimer’s disease. Expert Rev Neurother. 2007;7:887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid EB., Jr Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34(1–2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, Martin PR. Clinical assessment of cognition in alcoholism. Alcohol Clin Exp Res. 1986;10:123–127. doi: 10.1111/j.1530-0277.1986.tb05058.x. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Developmental chlorpyrifos causes behavioral and neurochemical defects in zebrafish. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2009.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallothionein knockout mice. Neurotoxicol Teratol. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- Facci L, Stevens DA, Skaper SD. Glycogen synthase kinase-3 inhibitors protect central neurons against excitotoxicity. Neuroreport. 2003;14:1467–1470. doi: 10.1097/00001756-200308060-00012. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neuro-degeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N’Gouemo P, Riaz A. Ethanol and neurotransmitter interactions—from molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TF. Introduction to quantitative genetics. 4th ed. Essex, England: Addison Wesley Longman Ltd; 1996. [Google Scholar]
- Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, et al. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement. 2008;4:223–227. doi: 10.1016/j.jalz.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- French RL, Heberlein UH. Glycogen Synthase Kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. doi: 10.1073/pnas.0910813106. PNAS; in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Tanaka N, Miki K, Hossain MN, Endoh M, Ayusawa D. Uncoupling of longevity and paraquat resistance in mutants of the nematode Caenorhabditis elegans . Biosci Biotechnol Biochem. 2005;69:2015–2018. doi: 10.1271/bbb.69.2015. [DOI] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, et al. Heritability for Alzheimer’s disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- Gatz M, Svedberg P, Pedersen NL, Mortimer JA, Berg S, Johansson B. Education and the risk of Alzheimer’s disease: findings from the study of dementia in Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2001;56:P292–P300. doi: 10.1093/geronb/56.5.p292. [DOI] [PubMed] [Google Scholar]
- Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Environmental pesticide exposure as a risk factor for Alzheimer’s disease: a case-control study. Environ Res. 2001;86:37–45. doi: 10.1006/enrs.2001.4254. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int. 2002;40:493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Gieseler K, Grisoni K, Segalat L. Genetic suppression of phenotypes arising from mutations in dystrophin-related genes in Caenorhabditis elegans . Curr Biol. 2000;10:1092–1097. doi: 10.1016/s0960-9822(00)00691-6. [DOI] [PubMed] [Google Scholar]
- Gilliam DM, Mantle MA, Barkhausen DA, Tweden DR. Effects of acute prenatal ethanol administration in a reciprocal cross of C57BL/6J and short-sleep mice: maternal effects and nonmaternal factors. Alcohol Clin Exp Res. 1997;21:28–34. [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol. 2003;25:335–347. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcy D, Ostbye T, Breitner JC, Welsh-Bohmer KA. Occupational exposures to pesticides increases the risk of Alzheimer’s disease: findings from the Cache County Study; Vienna. International Conference on AD Meetings (ICAD); 2009. Jul, pp. 10–16. [Google Scholar]
- Heyman A, Wilkinson WE, Stafford JA, Helms MJ, Sigmon AH, Weinberg T. Alzheimer’s disease: a study of epidemiological aspects. Ann Neurol. 1984;15:335–341. doi: 10.1002/ana.410150406. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Possidente D, Averill S, Despain TP, Buytkins J, Thomas V, et al. Variations at quantitative trait locus (QTL) affect development of behavior in lead-exposed Drosophila melanogaster . Neurotox. 2009;30(2):305–311. doi: 10.1016/j.neuro.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HVB, Mercer J, Sambaziotis H, Huber M, Stark DT, Torno-Morley T, et al. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster . Neurotox. 2003;24:435–442. doi: 10.1016/S0161-813X(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Hobert O. Specification of the nervous system. Worm Book. 2005 p:1–19. doi: 10.1895/wormbook.1.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Holbert S, Dedeoglu A, Humbert S, Saudou F, Ferrante RJ, Neri C. Cdc42-interacting protein 4 binds to Huntingtin: neuropathologic and biological evidence for a role in Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:2712–2717. doi: 10.1073/pnas.0437967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme SE, Shevkoplyas SS, Samuel A. Microfluidics: streamlining discovery in worm biology. Nat Methods. 2008;5:589–590. doi: 10.1038/nmeth0708-589. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ishii N, Takahashi K, Tomita S, Keino T, Honda S, Yoshino K, et al. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans . Mutat Res. 1990;237:165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, et al. Human wild-type tau interacts with wingless pathway components and produces neu-rofibrillary pathology in Drosophila. Neuron. 2002;34:509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jansen RC, Nap JP. Genetical genomics: the added value from segregation. Trends Genet. 2001;17(7):388–391. doi: 10.1016/s0168-9525(01)02310-1. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Seidler FJ, Slotkin TA. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chlorpyrifos. Brain Res Bull. 1998;45:143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- Jones D, Candido EP. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: relationship to the cellular stress response. J Exp Zool. 1999;284:147–157. doi: 10.1002/(sici)1097-010x(19990701)284:2<147::aid-jez4>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio) J Comp Neurol. 2001;440:342–377. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- Kaye JA Oldest-old healthy brain function. The genomic potential. Arch Neurol. 1997;54:1217–1221. doi: 10.1001/archneur.1997.00550220027009. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Quaife CJ, Froelick GJ, Palmiter RD. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Apolipoprotein E {epsilon}4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: the Cache County Study. Arch Gen Psychiatry. 2004;61:518–524. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sayato-Suzuki J. Zinc and copper accumulation and isometallothionein induction in mouse ascites sarcoma S180A cells. Biochem J. 1988;249:69–75. doi: 10.1042/bj2490069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE. Alcohol, nitric oxide, and neurotoxicity: is there a connection? A review Alcohol Clin Exp Res. 1992;16:539–541. doi: 10.1111/j.1530-0277.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;4:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, et al. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol. 2007;64:501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cerutti DT. Behavioral neuroscience of zebrafish. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. New York: CRC Press; 2008. pp. 293–310. [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis D, Yacisin E, Linney EK. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006a;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Perraut D, Pollard C, Freedman JHN. Metallothionein expression and neurocognitive function in mice. Physiol Behav. 2006b;87:513–518. doi: 10.1016/j.physbeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm G. The genome of Drosophila melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas JW, Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J Neurosci. 2005;25:4118–4126. doi: 10.1523/JNEUROSCI.4273-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF, Anholt RR. Ain’t misbehavin’? Genotype-environment interactions and the genetics of behavior Trends Genet. 2007;23(7):311–314. doi: 10.1016/j.tig.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Martin PR, Adinoff B, Weingartner H, Mukherjee AB, Eckardt MJ. Alcoholic organic brain disease: nosology and pathophysiologic mechanisms. Prog Neuropsychopharma-col Biol Psychiatry. 1986;10:147–164. doi: 10.1016/0278-5846(86)90069-2. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Genetic epidemiology of Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S58–S62. doi: 10.1097/00002093-200607001-00008. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradre-nergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Morley EJ, Hirsch HV, Hollocher K, Lnenicka GA. Effects of chronic lead exposure on the neuromuscular junction in Drosophila larvae. Neurotox. 2003;24(1):35–41. doi: 10.1016/s0161-813x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002a;26:547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002b;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neuro-degeneration in the developing brain. Apoptosis. 2000;5:515–521. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere JI, konomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Page GP, Ruden DM. In: Combining high dimensional biological data to study complex diseases and quantitative traits— design, analysis, and interpretation of results. Allison P, Beasley Edwards, editors. Boca Raton, FL: Chapman & Hall/CRC; 2005. [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. PKA-RII-deficient Drosophila are viable but show developmental, circadian and drug response phenotypes. J Biol Chem. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Parker JA, Connolly JB, Wellington C, Hayden M, Dausset J, Neri C. Expanded poly-glutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc Natl Acad Sci USA. 2001;98:13318–13323. doi: 10.1073/pnas.231476398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto NC, Roza T, Morsch VM, Pereira ME. Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci. 2007;25:39–46. doi: 10.1016/j.ijdevneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance MA, Grubber J, Bailey LR, Hedges D, West S, Santoro L, et al. Identification of novel genes in late-onset Alzheimer’s disease. Exp Gerontol. 2000;35:1343–1352. doi: 10.1016/s0531-5565(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Perls T. Genetic and environmental influences on exceptional longevity and the AGE nomogram. Ann NY Acad Sci. 2002;959:1–13. doi: 10.1111/j.1749-6632.2002.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SC. Cerebellar white matter after long-term ethanol consumption in mice. J Stud Alcohol. 1990;51:14–18. doi: 10.15288/jsa.1990.51.14. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Steffens DC, Burke JR, Welsh-Bohmer KA, Newman TN, Drosdick D, et al. Duke twins study of memory in aging in the NAS-NRC twin registry. Twin Res Hum Genet. 2006;9:950–957. doi: 10.1375/183242706779462381. [DOI] [PubMed] [Google Scholar]
- Portala K, Levander S, Westermark K, Ekselius L, von Knorring L. Pattern of neurop-sychological deficits in patients with treated Wilson’s disease. Eur Arch Psychiatry Clin Neurosci. 2001;251:262–268. doi: 10.1007/pl00007543. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ruan QL, Ju JJ, Li YH, Liu R, Pu YP, Yin LH, et al. Evaluation of pesticide toxicities with differing mechanisms using Caenorhabditis elegans . J Toxicol Environ Health A. 2009;72:746–751. doi: 10.1080/15287390902841532. [DOI] [PubMed] [Google Scholar]
- Ruden DM. Personalized medicine and quantitative trait transcripts. Nat Genet. 2007;39(2):144–145. doi: 10.1038/ng0207-144. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kurz M, Kemmler G, Mair D, Hausmann A, Hinterhuber H, et al. Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res. 2003;27:432–439. doi: 10.1097/01.ALC.0000057945.57330.2C. [DOI] [PubMed] [Google Scholar]
- Ryder E, Ashburner M, Bautista-Llacer R, Drummond J, Webster J, et al. The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics. 2007;177(1):615–629. doi: 10.1534/genetics.107.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez M, Bolumar F, Garcia AM. Occupational risk factors in Alzheimer’s disease: a review assessing the quality of published epidemiological studies. Occup Environ Med. 2007;64:723–732. doi: 10.1136/oem.2006.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz S, Fischer S, Guündel U, Kuüster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment-applications beyond acute toxicity testing. Environ Sci Pollut Res Int. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Luke CJ, Bhatia SR, Long OS, Vetica AC, Perlmutter DH, et al. Modeling molecular and cellular aspects of human disease using the nematode. Caenorhabditis elegans. 2008 doi: 10.1203/PDR.0b013e31819009b0. Pediatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila . Alcohol Clin Exp Res. 2000;24:1127–1136. [PubMed] [Google Scholar]
- Skoog I. Status of risk factors for vascular dementia. Neuroepidemiology. 1998;17:2–9. doi: 10.1159/000026147. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catechola-mine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;287:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans . Dev Biol. 1983;100:164–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sulston JE. Neuronal cell lineages in the nematode Caenorhabditis elegans . Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):443–452. doi: 10.1101/sqb.1983.048.01.049. [DOI] [PubMed] [Google Scholar]
- Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, et al. Ethanol and oxidative stress. Alcohol Clin Exp Res. 2001;25:237S–243S. doi: 10.1097/00000374-200105051-00038. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Switzer R, III, Majchrowicz E, Weight FF. Ethanol-induced aggyrophilia in entorhinal cortec of rat. Anat Rec. 1982;202:186a. [Google Scholar]
- Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur J Pharmacol. 2004;499:239–245. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- Takadera T, Sakamoto Y, Ohyashiki T. NMDA receptor 2B-selective antagonist ifen-prodil-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Brain Res. 2004;1020:196–203. doi: 10.1016/j.brainres.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K. Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc Natl Acad Sci USA. 1993;90:7789–7793. doi: 10.1073/pnas.90.16.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36(3):283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, et al. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008;30:38–45. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–597. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR. Alcohol abuse and alcoholism: an overview. J Clin Psychiatry. 2001;62(Suppl.20):4–10. [PubMed] [Google Scholar]
- Warren KR, Li TK. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. 2005;73:195–203. doi: 10.1002/bdra.20125. [DOI] [PubMed] [Google Scholar]
- Weber DN. Dose-dependent effects of developmental mercury exposure on C-start escape responses of larval zebrafish Danio rerio . J Fish Biol. 2006;69:65–94. [Google Scholar]
- Weight FF, Peoples RW, Wright JM, Lovinger DM, White G. Ethanol action onexcitatory amino acid activated ion channels. Alcohol Alcohol Suppl. 1993;2:353–358. [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci USA. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, et al. Antioxidant intake and cognitive function of elderly men and women: the cache county study. J Nutr Health Aging. 2007;11:230–237. [PubMed] [Google Scholar]
- Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav Process. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Poelchen W, Koles L, Muhlberg K, Scheibler P, Allgaier C, et al. Ethanol-induced inhibition of NMDA receptor channels. Neurochem Int. 1999;35:153–162. doi: 10.1016/s0197-0186(99)00057-1. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate modelsofdrug abuse. J Neurobiol. 2003;54:161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int. 1999;35:107–113. [PubMed] [Google Scholar]
- Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, et al. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans . J Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J Comp Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Xing X, Guo Y, Wang D. Using the larvae nematode Caenorhabditis elegans to evaluate neurobehavioral toxicity to metallic salts. Ecotoxicol Environ Safety. 2009 doi: 10.1016/j.ecoenv.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Yang W, Li J, Hekimi S. A measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans . Genetics. 2007;177:2063–2074. doi: 10.1534/genetics.107.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett. 2005;155:361–368. doi: 10.1016/j.toxlet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]