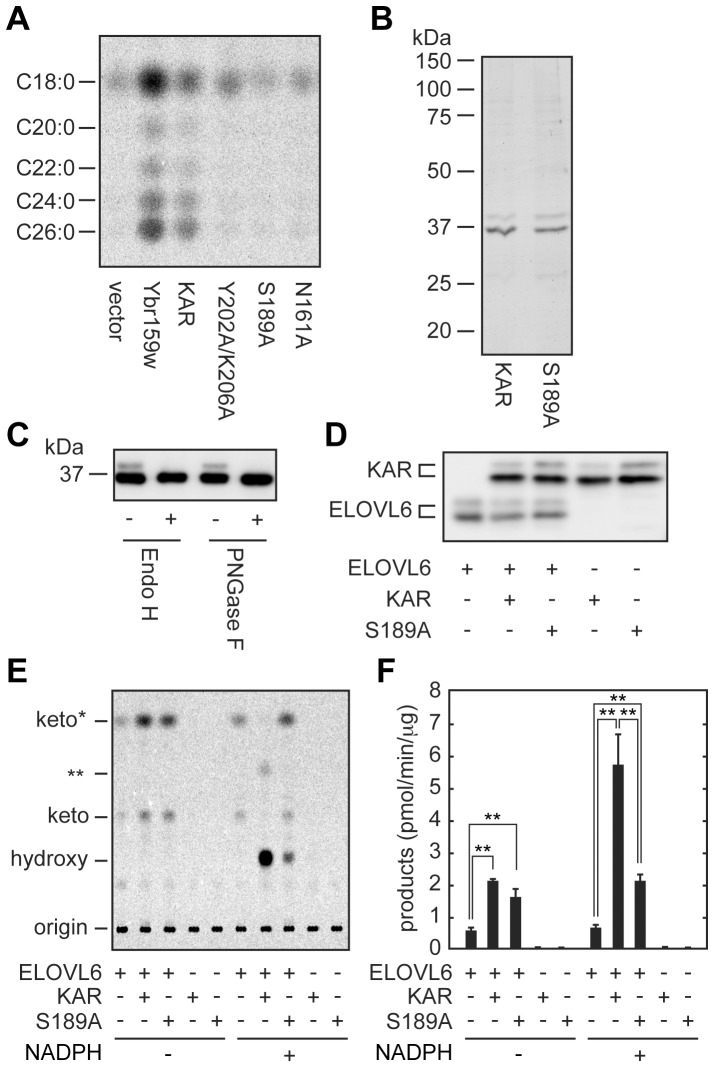
Figure 4. KAR stimulates ELOVL6 enzyme activity in KAR enzyme activity-independent and -dependent manners.
(A) The yeast TatY56 (ayr1Δ::natNT2 YBR159W-FLAG-AID) cells harboring the pWK40 (2xHA vector), pTN41(2xHA-YBR159w), pTN42 (2xHA-KAR), pTN44 (2xHA-KAR Y202A/K206A), pTN45 (2xHA-KAR S189A), or pTN46 (2xHA-KAR N161A) plasmid were treated with 0.5 mM 3-indolacetic acid for 2 h. Total membrane proteins (20 µg) prepared from them were incubated with 20 µM C16∶0-CoA, 73 µM malonyl-CoA, and 27 µM [14C]malonyl-CoA (0.075 µCi) for 30 min at 37°C in the presence of 1 mM NADPH. After termination of the reactions, lipids were saponified, acidified, extracted, converted to methyl ester forms, separated by reverse-phase TLC, and detected by a bioimaging analyzer BAS-2500. (B) Total membrane fractions prepared from HEK 293T cells transfected with the pCE-puro 3xFLAG-KAR or pCE-puro 3xFLAG-KAR S189A plasmid were solubilized with 2% Triton X-100, and subjected to affinity-purification using anti-FLAG M2 agarose affinity beads. Purified proteins (1 µg) were separated by SDS-PAGE and stained with Coomassie brilliant blue. (C) Purified 3xFLAG-KAR proteins (15 ng) were treated with Endo H or PNGase F and subjected to immunoblotting using an anti-FLAG M2 antibody. (D-F) Purified 3xFLAG-ELOVL6 (10 ng) and/or 3xFLAG-KAR proteins of either wild type or S189A mutant (20 ng) were reconstituted into proteoliposomes and subjected to immunoblotting using an anti-FLAG M2 antibody (D) or to an in vitro FA elongation assay (E and F). (E) Proteoliposomes were incubated with 8 µM C16∶0-CoA and 27.3 µM [14C]malonyl-CoA (1.5 µCi/ml) for 90 min at 37°C in the presence or absence of 1 mM NADPH. After termination of the reactions, lipids were saponified, acidified, extracted, and separated by normal-phase TLC, followed by detection using a bioimaging analyzer BAS-2500. keto, 3-keto-FA; *keto, a by-product of 3-ketoacyl-CoA; hydroxy, 3-hydroxy-FA; **, trans-2-enoyl-FA produced through non-enzymatic conversion or produced by contaminated 3-hydroxyacyl-CoA dehydratase. (F) Values presented are the sum of the reaction products in (E) and represent the mean ± S.D. from three independent experiments. Statistically significant differences are indicated (**p<0.01; t-test).