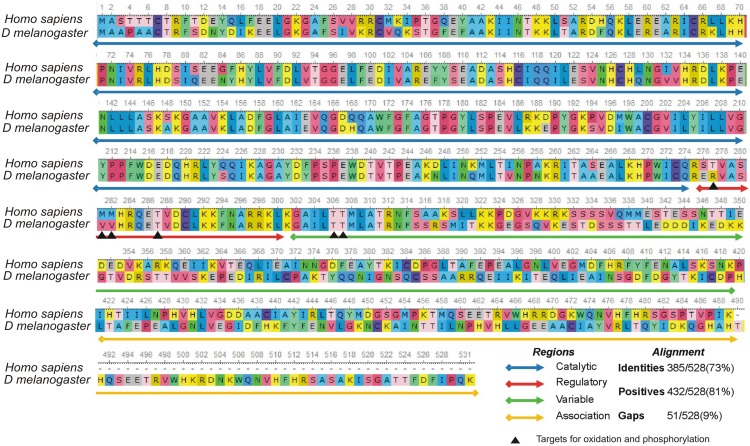
Figure 4. CaMKII from human and Drosophila melanogaster are highly conserved.
Alignment between CaMKIIδ from Homo sapiens and the corresponding enzyme in Drosophila melanogaster show conserved regions and residues of interest that are targets of phosphorylation. Thr287, Thr306 and Thr307 on human isoform have their homologous residues in Drosophila melanogaster. This might allow Drosophila melanogaster protein to be regulated by autophosphorylation like occurs in its human homologous enzyme. Unlike phosphorylation sites, Met281/282 oxidation sites are replaced by valine residues in the fruit fly, thus, regulation by oxidation of Met281/282, are absent in Drosophila melanogaster.