Summary
Several methicillin resistance (SCCmec) clusters characteristic of hospital-associated methicillin-resistant Staphylococcus aureus (MRSA) strains harbor the psm-mec locus. In addition to encoding the cytolysin, phenol-soluble modulin (PSM)-mec, this locus has been attributed gene regulatory functions. Here we employed genome-wide transcriptional profiling to define the regulatory function of the psm-mec locus. The immune evasion factor protein A emerged as the primary conserved and strongly regulated target of psm-mec, an effect we show is mediated by the psm-mec RNA. Furthermore, the psm-mec locus exerted regulatory effects that were more moderate in extent. For example, expression of PSM-mec limited expression of mecA, thereby decreasing methicillin resistance. Our study shows that the psm-mec locus has a rare dual regulatory RNA and encoded cytolysin function. Furthermore, our findings reveal a specific mechanism underscoring the recently emerging concept that S. aureus strains balance pronounced virulence and high expression of antibiotic resistance.
Keywords: Staphylococcus aureus, MRSA, virulence, antibiotic resistance
Introduction
Staphylococcus aureus is a dangerous human pathogen, which produces a series of toxins and other factors to avoid elimination by human innate host defense (Lowy, 1998). Resistance to efficient anti-staphylococcal antibiotics such as the penicillins and methicillin further complicates treatment of infections with virulent S. aureus (Lowy, 2003). Methicillin resistance is encoded on mobile genetic elements called staphylococcal cassette chromosome (SCC) mec (Hiramatsu et al., 2001). Several types of SCCmec exist. Recently, we discovered that SCCmec types II, III, and VIII, found in a large number of epidemic hospital-associated MRSA strains, contain a gene encoding a potent cytolysin of the phenol-soluble modulin (PSM) family, called PSM-mec (Chatterjee et al., 2011; Queck et al., 2009). PSM-mec is encoded adjacent to the mecA/mecR/mecI methicillin resistance gene cluster. The PSM-mec peptide efficiently lyses human neutrophils and erythrocytes and has pro-inflammatory capacities (Queck et al., 2009), similar to several other members of the PSM family (Cheung et al., 2010; Wang et al., 2007). Thus, in a way not previously described for S. aureus, psm-mec-encoding SCCmec elements allow for simultaneous acquisition of antibiotic resistance and virulence determinants, representing an important element for the evolution of S. aureus as a highly successful pathogen (Otto, 2010b).
However, there have been contrasting results regarding the contribution of psm-mec to MRSA virulence. We previously showed that, unless overshadowed by strong production of other PSMs, the PSM-mec toxin has a significant contribution to MRSA virulence (Chatterjee et al., 2011; Queck et al., 2009). Accordingly, we found that the psm-mec locus increases virulence or has no effect, dependent on which strain is used (Chatterjee et al., 2011). In contrast, Kaito et al. reported that psm-mec has gene regulatory functions that ultimately decrease, rather than increase, MRSA virulence. First, they described and attributed gene regulatory functions to an open reading frame (ORF) called “fudoh”, which shows considerable overlap with psm-mec, but is encoded in the opposite direction (Kaito et al., 2008). They later found that the psm-mec locus has regulatory functions that are independent of the PSM-mec peptide (Kaito et al., 2011), notably including down-regulation of expression of the strongly cytolytic PSMα peptides (Wang et al., 2007). Rather than to “fudoh”, this was then attributed to a psm-mec-encoded RNA (Kaito et al., 2011). Recently, they reported that the psm-mec RNA interacts with the agrA transcript, thereby decreasing production of the PSM regulator AgrA (Queck et al., 2008), but only in some strains (Kaito et al., 2013). Accordingly, in strains that showed a psm-mec-dependent difference in AgrA expression, production of PSMα peptides was increased, in selected strains reaching a maximum of about factor four (Kaito et al., 2013). In three strains, in which this effect was most pronounced, an impact on virulence was observed. Despite the overall minor and highly strain-dependent effect of psm-mec on PSMα production and virulence, these authors concluded that absence of the psm-mec locus is responsible for the increased virulence potential of community- versus hospital-associated MRSA in a general fashion (Kaito et al., 2013).
To analyze the regulatory impact of the psm-mec locus in detail, we here performed genome-wide transcriptional profiling of two psm-mec mutants in comparison with the corresponding isogenic MRSA wild-type strains. Furthermore, we produced single nonsense basepair mutations in the psm-mec and (hypothetical) “fudoh” start codons to determine whether gene regulatory functions are due to the PSM-mec peptide, a putative psm-mec regulatory RNA, or “fudoh”. Notably, these mutations were produced in the genome of psm-mec-containing MRSA strains to analyze regulatory functions in the natural strain background, without an impact of plasmid-based artificial over-expression. Our results emphasize the strain dependence of psm-mec regulation, but identify protein A as the only target of psm-mec that is strong in extent and conserved in at least a series of psm-mec-positive MRSA strains.
Materials and methods
Bacterial strains, plasmids, and growth conditions
The bacterial strains used in the present study are shown in Table. 1. MRSA252 is an epidemic SCCmec type II strain from the United Kingdom. MSA820, MSA890, MSA1601, and MSA3407 are MRSA strains recovered in the 1980s from Rhode Island (MSA820, MSA1601), Texas (MSA890) and New York (MSA3407), respectively (Fitzgerald et al., 2001; Musser and Kapur, 1992), also of SCCmec type II (Queck et al., 2009). All bacteria were grown in tryptic soy broth (TSB). A single base mutation of the start codon was introduced into psm-mec (resulting in psm-mec*) and into fudoh (resulting in fudoh*), with a procedure based on allelic replacement using plasmid pKOR1 (Bae and Schneewind, 2006). Growth patterns of the mutants in TSB were unchanged compared to the wild-type strain (Fig. 1). The agr mutant of strain MSA3407 was produced by phage transduction from strain RN6911, as described (Wang et al., 2007) and its psm-mec mutant was produced as described (Queck et al., 2009). For the construction of complementation plasmids, regions of DNA containing the respective promoter and gene were amplified from S. aureus genomic DNA of strain MSA3407, and cloned into plasmid pT181 via the EcoRI/BamHI sites. All oligonucleotides were synthesized by Sigma (Table 2).
Table 1.
Bacterial strains used in the present study.
| Strain | Antibiotic | Source |
|---|---|---|
| S. aureus MRSA252 | (Queck et al., 2009) | |
| S. aureus MRSA252 Δpsm-mec | (Queck et al., 2009) | |
| S. aureus MSA820 | (Queck et al., 2009) | |
| S. aureus MSA820 Δpsm-mec | (Queck et al., 2009) | |
| S. aureus MSA890 | (Queck et al., 2009) | |
| S. aureus MSA890 Δpsm-mec | (Queck et al., 2009) | |
| S. aureus MSA1601 | (Queck et al., 2009) | |
| S. aureus MSA1601 Δpsm-mec | (Queck et al., 2009) | |
| S. aureus MSA3407 | (Queck et al., 2009) | |
| S. aureus MSA3407 Δpsm-mec | (Queck et al., 2009) | |
| S. aureus MSA890 psm-mec* | This study | |
| S. aureus MSA890 fudoh* | This study | |
| S. aureus MSA3407 psm-mec* | This study | |
| S. aureus MSA3407 fudoh* | This study | |
| S. aureus MSA3407 (pT181) | Tet 12.5 μg/ml | This study |
| S. aureus MSA3407 (pTpsm-mec) | Tet 12.5 μg/ml | This study |
| S. aureus MSA3407 (pTpsm-mec*) | Tet 12.5 μg/ml | This study |
Fig. 1.
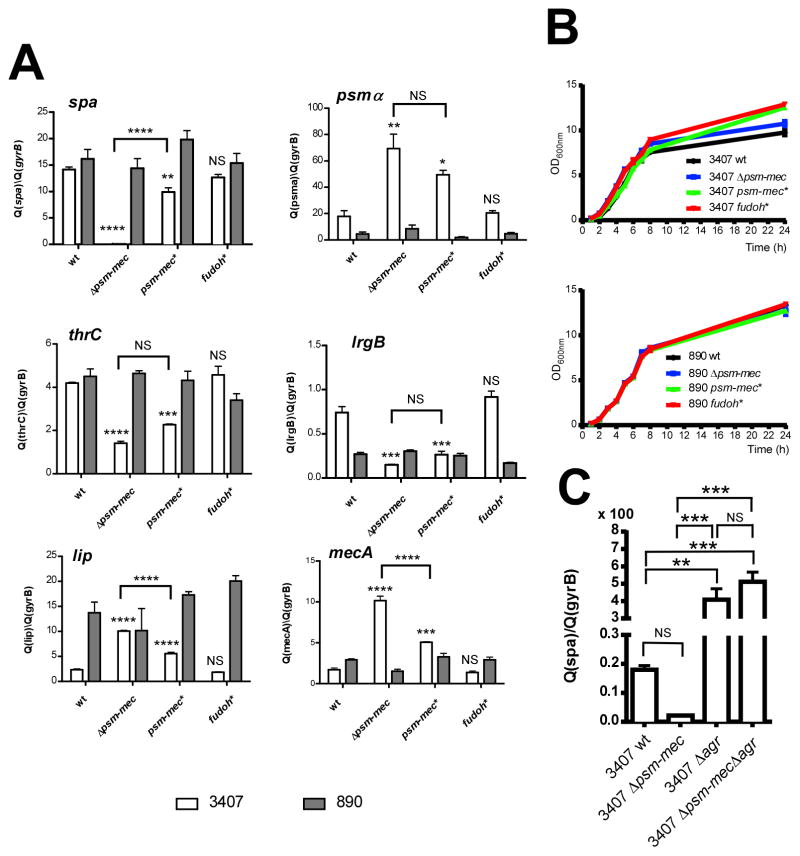
Effects of psm-mec on virulence gene expression. (A) Expression of selected virulence-associated genes in wild-type, psm-mec deletion and psm-mec and fudoh start codon deletion mutants in strains MSA3407 and MSA890. wt, wild-type strain; Δpsm-mec, psm-mec gene deletion strain; psm-mec*, strain harboring a psm-mec start codon mutation abolishing translation; fudoh*, fudoh (hypothetical) start codon mutation abolishing translation of hypothetical Fudoh protein. (B) Corresponding growth curves. (C) Effect of agr and agr/psm-mec deletion on spa transcription in strain MSA3407. (A, C) Gene expression was determined using qRT-PCR in late exponential phase (8-h) cultures. Values are from measurements performed at least in triplicate (three biological replicates, measured three times each) and relative to expression of the housekeeping gene gyrB. **, p<0.01; ***, p<0.001; ****, p<0.0001.
Table 2.
Oligonucleotides used in the present study.
| Oligonucleotide name | Oligonucleotide sequence |
|---|---|
| Construction of start codon mutation in fudoh | |
| MecFw | GGGGACAAGTTTGTACAAAAAAGCAGGCTTTAATGGCATTCGACCAAAA |
| MecRv | GGGGACCACTTTGTACAAGAAAGCTGGGTAACGCTGAAAGATTCGCTTC |
| MecFwmut | GTTGAAAAAATTAACCGAAAGCCTGAATACAAGTCTTG |
| MecRvmut | CAAGACTTGTATTCAGGCTTTCGGTTAATTTTTTCAAC |
| Oligonucleotides to test for presence of start codon mutation in fudoh | |
| MecFwseq | GGCGGTTTCAATTCACTTGT |
| PSM-mec, PSM-mec* and fudoh* complementation plasmids | |
| F-eco | ATGCGAATTCTATTTTATTTTCCATAATTGCCTACCCCATAAG |
| F-Bam | ATGCGGATCCCAATTCACTTGTCTTAAACTTTGTAGAAAAAGAAG |
| 5′ RACE | |
| PSM-mec GSP6 | AAACAATCAAGTCGTTGAATATTTCCTCTG |
| PSM-mec GSP7 | ATTAACCGAAAGCCTGAATGCAAGTCTTGAT |
| 3′ RACE | |
| PSM-mec GSP3 | ATGGATTTCACTGGTGTTATTAC |
| E1 | 5′-phosphate-UUCACUGUUCUUAGCGGCCGCAUGCUC-idT-3′ |
| E4 | GAGCATGCGGCCGCTAAGAACAGTGAA |
| qRT-PCR | |||
|---|---|---|---|
| Gene | Forward | Reverse | Probe |
| lip | ACCGCCCCAATAATGAGCTA | AGTGCATGGTTTCAATGGGTTT | CACTGAAGGATTAATATC |
| thrC | GGTAATGGCTGTGACAAAAGCAA | TTTCCAGTCGAAGCGCATATT | AGAGCAAGGTAAG AAAA |
| lrgB | AGGCGAAGTCGAAAAAGTAGGA | ACCGGCTGGTACGAAGAGTAAG | CGACACTAACAAATAAC |
| spa | CAGCAAACCATGCAGATGCTA | GCTAATGATAATCCACCAAATACAGTTG | CATTACCAGAAACTGGTGAAGAAAATCCATTCATTG |
| psma | TATCAAAAGCTTAATCGAACAATTC | CCCCTTCAAATAAGATGTTCATATC | AAAGACCTCCTTTGTTTGTTATGAAATCTTATTTACCAG |
| mecA | TTCCACATTGTTTCGGTCTAAAATT | AATGCAGAAAGACCAAAGCATACA | CCACGTTCTGATTTTAAA |
| gyrB | CAAATGATCACAGCATTTGGTACAG | CGGCATCAGTCATAATGACGAT | AATCGGTGGCGACTTTGATCTAGCGAAAG |
Measurement of phenol-soluble modulins (PSMs)
PSM amounts were measured by reversed-phase high-pressure liquid chromatography/electrospray mass spectrometry (RP-HPLC/ESI-MS) using an 1100 HPLC system connected to an MSD Trap SL mass spectrometer (Agilent). Samples (100 μl) were injected onto a Zorbax SB-C8 2.3 × 30 mm column (Agilent) and a 0.1% trifluoroacetic acid (TFA) water/0.1% TFA acetonitrile gradient was applied as follows (flow rate, 0.5 ml/min); 10% acetonitrile from 0 to 2 min, 50% acetonitrile from 2 to 4 min, 50 to 100% acetonitrile linear gradient from 4 to 9 min, 100% acetonitrile from 9 to 13 min, 0% acetonitrile from 13 to 16 min. Relative PSM production was determined by measuring the peak area of the extracted ion chromatogram corresponding to multiply-charged m/z values of each PSM by using QuantAnalysis software (Agilent Technologies).
RNA isolation, transcriptional profiling, and quantitative RT-PCR
RNA was isolated as previously described using an RNeasy Mini Kit (Qiagen) as recommended in a standard protocol (Wang et al., 2007) from cultures grown in TSB to late exponential growth phase (8 h). Remaining DNA was removed using RNase-free DNase I (Amersham Biosciences). Removal of contaminant DNA was confirmed by PCR. The reaction product was cleaned up with an RNeasy mini column. cDNA was synthesized and labeled according to the manufacturer’s suggestions for Affymetrixantisense genome arrays (Affymetrix). A gel shift assay with NeutrAvidin (Pierce Biotechnology) was performed to estimate the labeling efficiency based on the instructions from Affymetrix. Biotinylated S. aureus cDNA was hybridized to custom Affymetrix GeneChips (RMLChip 3) with 96% coverage of genes from MW2 (2534 probe sets of 2632 ORFs) and scanned according to standard GeneChip protocols (Affymetrix). Each experiment was replicated at least 3 times. Affymetrix GeneChip Operating Software (GCOS v1.4, http://www.affymetrix.com) was used to perform the preliminary analysis of the custom chips at the probe-set level. Genes were regarded as differentially regulated when they passed significance tests and the factor of regulation was grater than two. The complete set of microarray data was deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and is accessible through GEO Series accession number GSE51366. Quantitative RT-PCR was performed as described (Wang et al., 2007).
Western blot analysis
Harvested bacterial pellets were washed with phosphate-buffered saline (PBS) and then resuspended in PBS to the same OD600nm. Cells were treated with lysostaphin for 5 min at 37°C and then centrifuged (13,000 rpm, 4°C, 5 min). Supernatants were mixed with 2 × SDS PAGE loading buffer and samples were heated for 5 min at 95°C. Protein bands were transferred from 12% bis/acrylamide SDS-PAGE gels onto nitrocellulose membranes using an iBlot Dry Blotting System (Invitrogen). Blots were incubated with anti-protein A antibody (Sigma) at 1:5,000 dilution and IRDye800CW-conjugated anti-rabbit antibody at 1:10,000 dilution. Signal were visulaized using an Odyssey Infrared Imaging System (LI-COR Biosciences). A positive control of staphylococcal surface protein A (Sigma) was used at 25 μg per lane. Membranes were washed in between incubations with Tris-buffered saline (pH 7.4, KD Medical) containing 0.1% Tween-20 (Sigma). All antibodies were diluted in Odyssey blocking buffer (Licor Biosciences).
Methicillin resistance
Resistance to methicillin was determined using oxacillin (Sigma) using a microtiter plate-based assay. Two hundred μl of a 2.048 mg/ml oxacillin solution was pipetted into a well, from which 100 μl were transferred to the next well. Then, 100 μl were transferred from that well to the next. This process was repeated another ten times. Then, 100 μl of a 1:100 dilution of a pre-culture grown overnight was added to each well, resulting in oxacillin concentrations ranging from one to 1024 μg/ml. Plates were grown for 24 h with shaking at 180 rpm at 37°C. Then, optical density at 600 nm was measured using an ELISA plate reader. The analysis was performed in quadruplicate (four wells per strain and concentration).
5′- and 3′-rapid amplification of cDNA ends (RACE)
5′- and 3′- RACE was performed to identify the full length of the psm-mec RNA transcript. 3′- RACE was performed as described previously (Argaman et al., 2001). Briefly, a 3′ RNA adapter, primer E1 was ligated to calf intestine alkaline phosphatase treated-RNA with T4 RNA ligase (New England Biolabs) according to the manufacturer’s instructions. Reverse transcription was performed with primer E4. PCR was performed using primers E4 and psm-mec GSP3. PCR products were purified and then sequenced with primer psm-mec GSP3. 5′- RACE was performed using the 5′- RACE System for Rapid Amplification of cDNA Ends Kit (Invitrogen). Primer PSM-mec GSP6 was used as the RNA adapter. PCR was performed using primers PSM-mec GSP6 and PSM-mec GSP7. PCR products were purified and then sequenced with PSM-mec GSP7. Mutations were confirmed by DNA sequencing.
Statistics
1-way ANOVA with Bonferroni’s post-tests or unpaired t tests were used to calculate 2-tailed P values using Graph Pad Prism 6 software. P < 0.05 was considered significant. Error bars depict mean ± SEM.
Results
To analyze the gene regulatory function of the psm-mec locus, we used two psm-mec- positive MRSA strains and their isogenic psm-mec deletion mutants in genome-wide transcriptional profiling (microarray) analyses. Strain MSA3407 is characteristic of the subset of PSM-mec-positive strains that produce high amounts of PSM-mec and other PSMs (Queck et al., 2009). The other, MRSA252, produces lower amounts of PSM-mec and other PSMs (Queck et al., 2009), due to the recently described agrC mutation in contemporary isolates of clonal complex 30 strains (DeLeo et al., 2011) and the fact that all PSMs including PSM-mec are regulated by Agr (Chatterjee et al., 2011; Queck et al., 2008). MRSA252 was included in the analysis because it is a genome-sequenced strain (Holden et al., 2004). Notably, we confirmed by DNA sequencing that none of the strains used in the present study contains the -7 T>C mutation in the psm-mec promoter shown by Kaito et al. to lead to absence of the suppression of AgrA and PSMα expression (Kaito et al., 2013).
According to the results of our microarray analyses, a series of genes were regulated by the psm-mec locus in the two strains (Tab. 3). The factors of regulation were overall higher in MSA3407 than MRSA252, most likely due to the fact that psm-mec is more strongly expressed in MSA3407 (Queck et al., 2009). However, the psm-mec regulons of the two strains only showed very limited similarity to each other; and the only gene with pronounced psm-mec-dependent expression changes that was detected in both regulons was the spa gene coding for protein A. Other strongly regulated genes comprised purine, sugar, and amino acid metabolism genes. However, these effects were not consistent among the tested strains. Of note, we did not detect genes of the Agr regulon, such as toxin genes, to be significantly changed in expression in the fashion that the results by Kaito et al. would suggest (Kaito et al., 2013). This suggests that the effects of psm-mec in this strain are not mediated via an effect on AgrA.
We then verified the microarray results using qRT-PCR, focusing on strain MSA3407, owing to the strong extent of psm-mec expression and observed psm-mec- dependent regulatory effects in that strain. In these analyses, we included the complete psm-mec deletion strain and single genomic basepair mutants abolishing translation of the psm-mec gene and the hypothetical “fudoh” gene (psm-mec* and fudoh* strains, respectively). The qRT-PCR results confirmed the extremely strong effect of the psm- mec locus on transcription of the spa gene (factor of ~ 175) and demonstrated that this effect was not due to the hypothetical protein product of the “fudoh” ORF (Fig. 1A). Importantly, spa expression levels in the psm-mec* strain with abolished production of the PSM-mec peptide were only slightly lower than those in the wild-type strain, indicating that the regulatory effect is largely due to the psm-mec RNA rather than the PSM-mec peptide. Furthermore, we attempted to analyze the direct, according to our microarray results, AgrA-independent effect of psm-mec on spa transcription using an agr mutant of strain MSA3407. However, we could not detect significant differences in spa transcript levels between the agr and agr/psm-mec deletion strains (Fig. 1C), likely because the effect of the agr deletion on spa is too strong in comparison. The overwhelming effect of agr became evident in the lack of significance computed by ANOVA for the wild-type vs. psm-mec comparison (compared to the significance attributed to the wild-type vs. agr or agr/psm-mec comparisons). Using a t-test directly comparing these two strains, the difference was, however, significant (p=0.0003), in accordance with the data shown in Fig. 1A.
Western blot analysis showed increased expression of protein A in the MSA3407 psm-mec wild-type compared to the psm-mec deletion strains, in accordance with up-regulation of the spa transcript (Fig. 2). We found similar effects on protein A production in strains MSA820 and MSA1601, while no difference was found between the MSA890 and MRSA252 wild-type and corresponding psm-mec deletion mutants. These results indicate that strong expression of psm-mec, as present in strains MSA3407, MSA1601, and MSA820, but not MSA890 and MRSA252 (Queck et al., 2009), is a prerequisite for an impact on protein A expression. Furthermore, they show that psm-mec RNA-dependent regulation is conserved among different S. aureus strains.
Fig. 2.
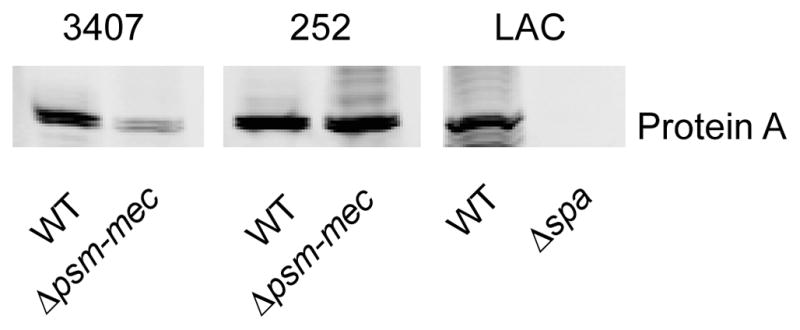
Western blot analysis of protein A expression. Surface protein preparations obtained by lysostaphin digestion from 8-h cultures grown in TSB were probed using a specific anti-protein A antibody. 3407, strain MSA3407; similar results were obtained with strains MSA1601 and MSA820. 252, strain MRSA252; similar results were obtained with strain 890. Strain LAC and isogenic Δspa (protein A) mutant were used as controls.
In addition, we analyzed by qRT-PCR a series of further mostly virulence-associated genes that showed psm-mec-dependent regulation in the microarray results. The effects of psm-mec on the expression of those genes were overall low and sometimes not significant. Notably, the hypothetical “Fudoh” protein did not have a significant impact on the expression of any of the investigated genes. In these assays, we also included the psmα operon, which according to Kaito et al. is regulated by the psm-mec locus independently of the PSM-mec peptide (Kaito et al., 2013; Kaito et al., 2011), and because the short psmα genes were not included in the microarray chip used. We could confirm that psmα transcripts were influenced in a PSM-mec peptide-independent manner. However, in accordance with our previous results (Chatterjee et al., 2011) and those of Kaito et al. (Kaito et al., 2013), this effect was minor (factor of ~ 3). Furthermore, measurement of PSM peptide production indicated that there was no considerable impact of the psm-mec locus, either by the psm-mec RNA, the PSM-mec peptide, or the hypothetical “Fudoh” protein, on the production of any PSM peptide (Fig. 3). As expected, PSM-mec production was abolished in the psm-mec deletion strain and unchanged in the fudoh* strain.
Fig. 3.
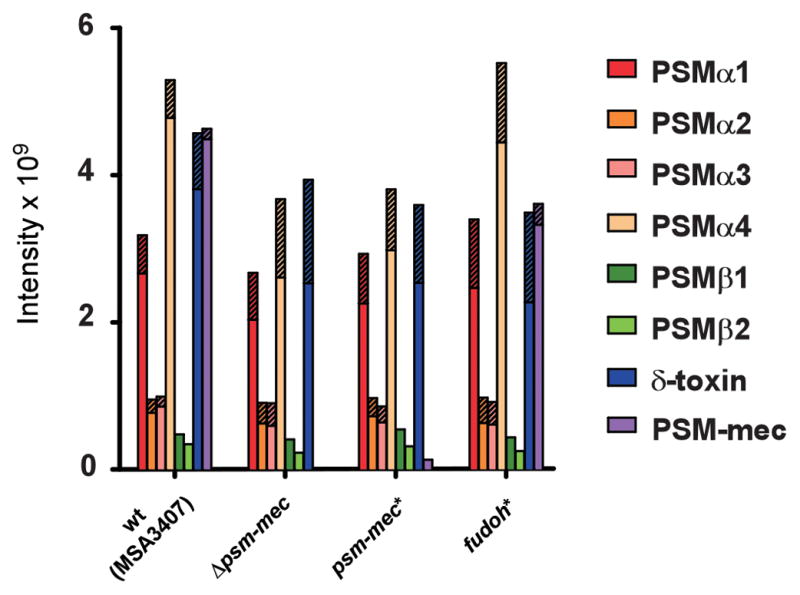
PSM production in MSA3407 wild-type, psm-mec deletion and psm-mec and fudoh start codon deletion mutants. Culture filtrates from 16-h cultures grown in TSB were analyzed for PSM production by HPLC/MS. Parts of bars with cross stripes represent N-deformylated portions. wt, wild-type strain; Δpsm-mec, psm-mec gene deletion strain; psm-mec*, strain harboring a psm-mec start codon mutation abolishing translation; fudoh*, fudoh (hypothetical) start codon mutation abolishing translation of hypothetical Fudoh protein.
Interestingly, we reproducibly detected very small amounts of PSM-mec peptide in the psm-mec* strain (Fig. 3), indicating that the mutated codon ATA may be used as non-canonical start codon, albeit in a strongly reduced fashion. While to our knowledge use of ATA as start codon has not been described in staphylococci, it was reported in other bacteria, for example E. coli (Kopke and Leggatt, 1991). The fact that the psm-mec gene is one of the most strongly translated genes in S. aureus may play a role for accepting a non-canonical start codon to a certain extent. This does not change the basis of our conclusions; it may only cause a slight under-estimation of the effect mediated by the PSM-mec peptide.
Notably, the psm-mec locus had a moderate, but significant negative impact on the expression of the neighboring mecA gene (~ 6-fold by qRT-PCR and ~ 2-fold by microarray in MSA3407; ~ 3-fold by microarray in MRSA252), apparently mediated at least in part via the PSM-mec peptide, suggesting that PSM-mec expression limits expression of methicillin resistance. Accordingly, resistance to oxacillin was increased significantly in the MSA3407 psm-mec deletion strain and the isogenic psm-mec* strain, compared to the corresponding wild-type strain (MICs: MSA3407, 49 μg/ml; psm-mec mutant, 80 mg/ml; psm-mec*, 87 μg/ml) (Fig. 4).
Fig. 4.
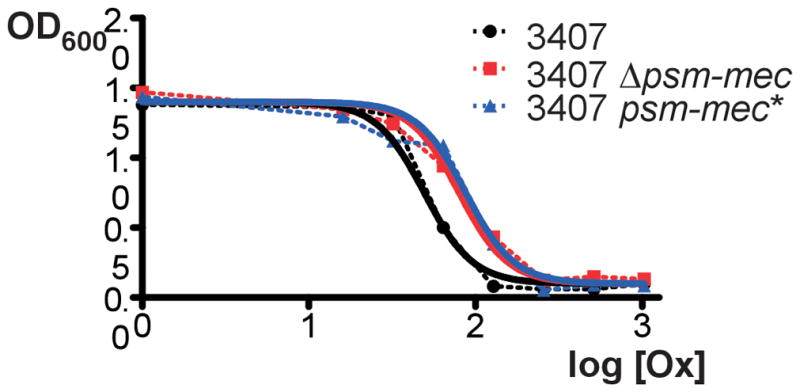
Impact of the psm-mec locus on oxacillin resistance. Resistance to oxacillin was measured in a microtiter plate assay in quadruplicate. 3407, MSA3407 wild-type strain; Δpsm-mec, psm-mec gene deletion strain; psm-mec*, strain harboring a psm-mec start codon mutation abolishing translation. Dotted lines show connecting curves; solid lines are non-linear best-fit calculated curves.
We also performed qRT-PCR analyses of the same genes as analyzed for the MSA3407 strain and derivatives using the psm-mec deletion, psm-mec*, and fudoh* mutants of strain MSA890 (Fig. 1). We showed previously that this strain only produces very low absolute, but high amounts of PSM-mec relative to those of other PSMs, resulting in a measurable virulence-enhancing effect of the psm-mec locus (Queck et al., 2009). In strain MSA890, we did not detect considerable effects on the expression of spa or any other of the selected genes, indicating that the psm-mec locus must be expressed at high levels for gene regulatory effects to occur, in accordance with the results we obtained on protein A production.
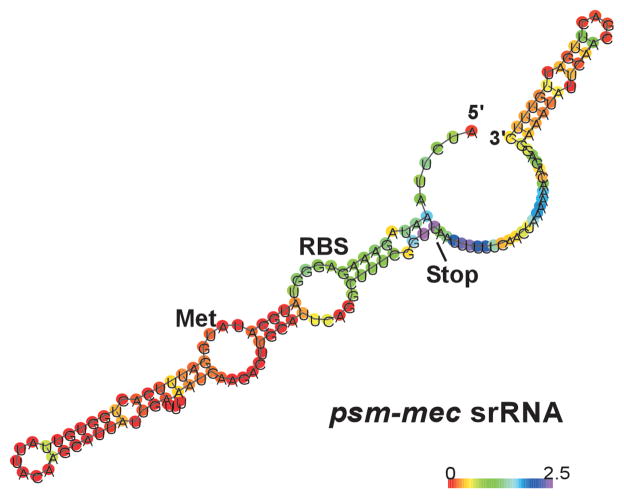
Together, our experiments identified the spa gene as the only conserved target of psm-mec-dependent regulation that is considerable in extent and occurs independently of the PSM-mec peptide. As our results indicated that the psm-mec gene is embedded within a regulatory RNA, we set out to precisely determine the extensions of that RNA. 5′- and 3′- RACE experiments showed that the psm-mec RNA only slightly extends from the boundaries of the psm-mec gene (Fig. 5), in accordance with the results obtained by Kaito et al. (Kaito et al., 2013; Kaito et al., 2011). To verify that this RNA has the regulatory capacity determined in the microarray and qRT-PCR experiments, we complemented the psm-mec deletion mutant of strain MSA3407 with a plasmid expressing the corresponding sequence (Fig. 6). This construct increased the low-level expression of spa of the mutant strain by about the same factor as detected by comparison of the MSA3407 wild-type and psm-mec deletion strains, confirming that this RNA confers the regulatory features described above. In addition, we produced constructs with single basepair mutations of the psm-mec gene, similar to those introduced in the genomes of strains MSA3407 and MSA890. Results with these constructs confirmed those obtained with the genomic mutants, inasmuch as abolished translation of psm-mec did not affect spa transcription. Finally, we performed qRT-PCR with the complementation clones also with the other selected genes (see Fig. 1). Clones with the plasmid expressing psm-mec showed results opposite to those shown in Fig. 1 in strains with deletion of psm-mec, confirming the results shown in Fig. 1 (Fig. 6). Furthermore, clones with plasmids containing the single basepair mutations of the psm-mec genes showed similar results, with no significant difference to the respective clones expressing the PSM-mec peptide, confirming that the effects are mediated by the psm-mec RNA.
Fig. 5.
The psm-mec RNA. The extensions of the psm-mec RNA were determined by 5′- and 3′-RACE. Secondary structure prediction was performed using the RNAfold Webserver at the University of Vienna (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi). Color bar shows basepair probability. The N-terminus (“Met”) and C-terminus (“Stop”) of the PSM-mec-encoding region are depicted.
Fig. 6.
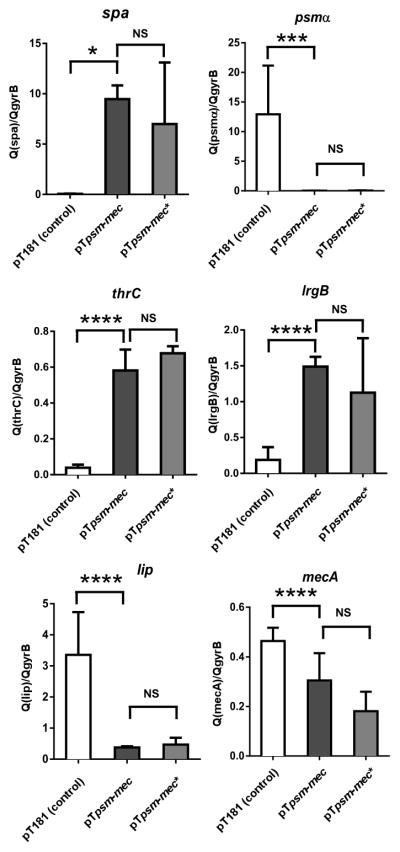
Plasmid-based complementation of the MSA3407 psm-mec deletion mutant with psm-mec and psm-mec*. Values are from measurements performed at least in triplicate (three biological replicates, measured three times each) and relative to expression of the housekeeping gene gyrB. *, p<0.05; ***, p<0.001; ****, p<0.0001.
Discussion
Results obtained in the present study reveal the spa gene encoding protein A as the primary conserved regulatory target of the psm-mec srRNA. Kaito et al. previously also noted psm-mec-dependent up-regulation of protein A expression in some strains. However, these authors attributed this effect to a general inhibition of the Agr system by the psm-mec RNA (Kaito et al., 2013). In contrast, our genome-wide transcriptional profiling analyses did not show gene regulatory changes, such as decreased production of toxins, that would be expected from an inhibition of AgrA translation and thus, functionality of the Agr system, as reported by Kaito et al. (Kaito et al., 2013). Of note, our findings do not challenge the results by Kaito et al., but the claim by those authors on the general applicability of their results and the conclusion that the mechanism they described would explain the previously noted increased virulence of CA- versus HA-MRSA (Kaito et al., 2013; Otto, 2010a). Rather, the impact of psm-mec on virulence appears to be highly strain-dependent.
We noted previously that the acquisition of psm-mec-containing SCCmec elements may simultaneously enhance virulence and antibiotic resistance properties in some MRSA strains such as MSA890 (Otto, 2010b). However, we also show here that expression of the PSM-mec peptide may lead to decreased expression of the mecA gene, resulting in decreased oxacillin resistance. This finding is in accordance with a recently emerging concept indicating that expression of methicillin resistance in MRSA strains is balanced versus virulence gene expression (Cheung et al., 2011; Rudkin et al., 2012). Possibly, considerable expression of both PBP2a and virulence determinants, PSM-mec in the present case, would cause too strong a metabolic burden for the bacteria.
Interestingly, the psm-mec locus has striking similarities with RNAIII, one of the best-studied srRNAs, which represents the intracellular regulatory effector molecule of the S. aureus accessory gene regulator (Agr) quorum-sensing system (Novick et al., 1993). Both the RNAIII and psm-mec srRNAs regulate the expression of virulence-associated genes and encode a cytolytic PSM peptide, PSM-mec in the case of the psm-mec srRNA and δ-toxin in the case of RNAIII. Based on the comparison of psm gene loci and their regulation, we proposed previously that RNAIII evolved around the hld gene coding for the PSM δ-toxin, thus linking quorum-sensing control of virulence genes to that of PSMs (Queck et al., 2008). The psm-mec srRNA might have evolved around the psm-mec gene in a similar fashion. Of note, the psm-mec locus so far represents the only known example, in addition to RNAIII, that combines virulence-enhancing properties mediated by an srRNA and an encoded protein. Together with the Escherichia coli srgS RNA (Wadler and Vanderpool, 2007), RNAIII and psm-mec form rare examples of srRNAs that also encode proteins.
The mechanism by which the psm-mec RNA controls the level of spa transcript is unclear. We speculated that it may bind to the spa promoter region; and bioinformatics analysis in fact suggested base pairing with the region upstream of the spa gene with the psm-mec RNA. However, electrophoretic mobility assays that we performed did not reveal specific binding (data not shown). Furthermore, in a previous report, the mapped spa promoter sequence did not include the region for which we detected basepairing in silico (Gao and Stewart, 2004).
A main function of protein A is to non-specifically bind immunoglobulins, thus producing a “camouflage coat” preventing opsonization via specific antibodies (Forsgren and Nordstrom, 1974). This control may lead to extended protection from antibody-mediated host defenses. However, it is in general debatable whether the acquired immune system plays a significant role in controlling S. aureus infection (Lin et al., 2009). Still, protein A has multiple functions in pathogenesis that are not dependent on evading the acquired immune defense (Gomez et al., 2004; Merino et al., 2009). We performed mouse models of bacteremia and pneumonia using the MSA3407 strain and its isogenic psm-mec mutant, in addition to the skin infection model performed previously (Queck et al., 2009), but were unable to detect significant differences (data not shown). Likely, the effect of psm-mec-dependent differential protein A expression on virulence may be too low and overshadowed by other virulence factors, such as α-toxin, leukocidins, and other PSMs.
Finally, it has remained unclear whether the gene product of the “fudoh” ORF first believed to be involved in gene regulatory effects attributed to the psm-mec locus (Kaito et al., 2008) participates in gene regulation. Our results using genomic and plasmid constructs with a mutated start codon abolishing “fudoh” translation clearly demonstrate that the hypothetical gene product of that ORF is not involved in the gene regulatory functions described here and elsewhere (Kaito et al., 2008; Kaito et al., 2011). Furthermore, it is highly likely that there is no “fudoh” gene or protein, given the wealth of data now available on the function of the overlapping psm-mec gene and its product, the PSM-mec peptide (Chatterjee et al., 2011; Kaito et al., 2008; Kaito et al., 2011; Queck et al., 2009).
In conclusion, we identified the spa gene as a conserved and strongly regulated target of the psm-mec locus. The negative impact of PSM-mec on the expression of mecA represents a specific mechanistic example underlining the recent concept that MRSA strains balance methicillin resistance versus virulence gene expression. However, our results also underline the strain dependence of the impact of psm-mec on virulence and methicillin resistance.
Table 3.
Genes strongly regulated by psm-mec (microarray analysis, all genes with up- or down-regulation by a factor > 10 are shown for MSA3407 and > 5 for MRSA252)1.
| Gene number2 | Function | Factor of regulation |
|---|---|---|
| MSA3407, up-regulated | ||
| 02548 (COL) | Protein A | 43.2 |
| SAV2377 (Mu50) | PTS system, sucrose-specific IIBC component | 20.7 |
| SAV2538 (Mu50) | PTS system, glucose-specific II ABC component | 16.4 |
| SAV2507 (Mu50) | Gluconate operon transcriptional repressor | 14.1 |
| SAV1068 (Mu50) | Phosphoribosylformylglycinamidine synthase I | 11.5 |
| SAV1065 (Mu50) | Phosphoribosylaminoimidazole carboxylase | 10.1 |
| MSA3407, down-regulated | ||
| SAV1330 (Mu50) | Homoserine kinase | 11.6 |
| SAV1329 (Mu50) | Threonine synthase | 11.3 |
| MRSA252, up-regulated | ||
| SAV2190 (Mu50) | PTS system, lactose-specific IIBC component | 10.0 |
| SAV2194 (Mu50) | Galactose-6-phosphate isomerase LacB subunit | 6.6 |
| 01835 (COL) | Tagatose-6-phosphate kinase (LacC) | 6.5 |
| 02548 (COL) | Protein A | 5.7 |
| MRSA252, down-regulated | ||
| SAV1071 (Mu50) | Phosphoribosylformylglycinamidine cyclo-ligase | 8.2 |
| SAV1069 (Mu50) | Phosphoribosylformylglycinamidine synthetase | 7.4 |
| 00597 (COL) | Phosphoribosylglycinamide formyltransferase | 7.1 |
| 708 (EMRSA16) | Phosphoribosylaminoimidazole- succinocarboxamide synthetase | 6.5 |
| SAV1068 (Mu50) | Phosphoribosylformylglycinamidine synthase I | 6.4 |
| SAV1073 (Mu50) | Bifunctional purine biosynthesis protein | 5.9 |
| SAV1074 (Mu50) | Phosphoribosylamine-glycine ligase | 5.4 |
| SAV1065 (Mu50) | Phosphoribosylaminoimidazole carboxylase | 5.3 |
The entire list of differentially regulated genes can be accessed using the GEO database (XXX).
Gene number according to strain Mu50 (Kuroda et al., 2001), unless gene not present in Mu50.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, U. S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e28781. doi: 10.1371/journal.pone.0028781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A. 2011;108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JR, Sturdevant DE, Mackie SM, Gill SR, Musser JM. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc Natl Acad Sci U S A. 2001;98:8821–8826. doi: 10.1073/pnas.161098098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A, Nordstrom K. Protein A from Staphylococcus aureus: the biological significance of its reaction with IgG. Ann N Y Acad Sci. 1974;236:252–266. doi: 10.1111/j.1749-6632.1974.tb41496.x. [DOI] [PubMed] [Google Scholar]
- Gao J, Stewart GC. Regulatory elements of the Staphylococcus aureus protein A (Spa) promoter. J Bacteriol. 2004;186:3738–3748. doi: 10.1128/JB.186.12.3738-3748.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–493. doi: 10.1016/s0966-842x(01)02175-8. [DOI] [PubMed] [Google Scholar]
- Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher C, Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, Mayes R, Moule S, Mungall K, Ormond D, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Sharp S, Simmonds M, Stevens K, Whitehead S, Barrell BG, Spratt BG, Parkhill J. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Omae Y, Matsumoto Y, Nagata M, Yamaguchi H, Aoto T, Ito T, Hiramatsu K, Sekimizu K. A novel gene, fudoh, in the SCCmec region suppresses the colony spreading ability and virulence of Staphylococcus aureus. PLoS One. 2008;3:e3921. doi: 10.1371/journal.pone.0003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Saito Y, Ikuo M, Omae Y, Mao H, Nagano G, Fujiyuki T, Numata S, Han X, Obata K, Hasegawa S, Yamaguchi H, Inokuchi K, Ito T, Hiramatsu K, Sekimizu K. Mobile genetic element SCCmec-encoded psm-mec RNA suppresses translation of agrA and attenuates MRSA virulence. PLoS Pathog. 2013;9:e1003269. doi: 10.1371/journal.ppat.1003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Saito Y, Nagano G, Ikuo M, Omae Y, Hanada Y, Han X, Kuwahara-Arai K, Hishinuma T, Baba T, Ito T, Hiramatsu K, Sekimizu K. Transcription and translation products of the cytolysin gene psm-mec on the mobile genetic element SCCmec regulate Staphylococcus aureus virulence. PLoS Pathog. 2011;7:e1001267. doi: 10.1371/journal.ppat.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke AK, Leggatt PA. Initiation of translation at an AUA codon for an archaebacterial protein gene expressed in E. coli. Nucleic Acids Res. 1991;19:5169–5172. doi: 10.1093/nar/19.19.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. Th1–Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penades JR, Lasa I. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol. 2009;191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser JM, Kapur V. Clonal analysis of methicillin-resistant Staphylococcus aureus strains from intercontinental sources: association of the mec gene with divergent phylogenetic lineages implies dissemination by horizontal transfer and recombination. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010a;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcus aureus toxin gene hitchhikes on a transferable antibiotic resistance element. Virulence. 2010b;1:49–51. doi: 10.4161/viru.1.1.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, Deleo FR, Otto M. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin JK, Edwards AM, Bowden MG, Brown EL, Pozzi C, Waters EM, Chan WC, Williams P, O’Gara JP, Massey RC. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J Infect Dis. 2012;205:798–806. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci U S A. 2007;104:20454–20459. doi: 10.1073/pnas.0708102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]