Abstract
Objective
The Rotterdam computed tomography (CT) score refined features of the Marshall score and was designed to categorize traumatic brain injury (TBI) type and severity in adults. The objective of this study was to determine whether the Rotterdam CT score can be used for mortality risk stratification after pediatric TBI.
Design
In children with moderate to severe TBI, a comparison of observed versus predicted mortality calculated using published model probabilities of adult mortality. Development and validation of a new pediatric mortality model using randomly selected prediction and validation samples from our cohort.
Setting
A single level 1 pediatric trauma center.
Subjects
632 children with moderate or severe TBI
Interventions
None.
Measurements and Main Results
Sixteen percent (101/632) of the patients died prior to hospital discharge. The predicted mortality based on Rotterdam score for adults with moderate or severe TBI discriminated pediatric observed mortality well (AUC = 0.85, 95% confidence interval [CI] 0.80 – 0.89) but had poor calibration, overestimating or underestimating mortality for children in several Rotterdam categories. A predictive model based on children with moderate or severe TBI from the single center discriminated mortality well (AUC 0.80, 95% CI 0.68 – 0.91) and showed good calibration and overall fit.
Conclusions
Children with TBI have better survival than adults in Rotterdam CT score categories representing less severe injuries, but worse survival than adults in higher score categories. A novel, validated pediatric mortality model based on the Rotterdam score is accurate in children with moderate or severe TBI and can be used for risk stratification.
Keywords: Pediatrics, Brain Injuries, Intensive Care Unit, Pediatric, Neuroradiography, Craniocerebral Trauma, Intracranial Hypertension
Background
Prognostic information is helpful for caregivers and family members to guide care of patients with traumatic brain injury (TBI), especially for those with life-threatening injuries. Because patients with severe TBI or hypoxia after TBI are frequently intubated and sedated(1–3), and often receive neuromuscular blocking agents prior to arrival in the emergency department, the initial neurologic exam may be limited. Radiographic imaging is one the earliest pieces of objective data available to evaluate severity of head injury and aid in determining prognosis. Non-contrast computed tomography (CT) scan of the head is the initial imaging study of choice due to its rapid image acquisition and ready availability in most hospitals.
The utility of CT imaging in predicting mortality and functional outcomes has been evaluated for both individual injury characteristics and composite scoring systems. Individual CT components that predict mortality or functional outcome include degree of midline shift (4–7), intraventricular hemorrhage (IVH) (4, 8), subarachnoid hemorrhage (SAH) (6, 7, 9, 10) and presence of cerebral edema.(4–7, 11, 12) Several of these studies included children in the evaluation of CT characteristics(4, 7, 8, 11, 12), although few focused exclusively on children. (7, 8, 11)
The Marshall score, developed in 1991 using the National Traumatic Coma Database, is one of the most frequently used CT scoring systems in TBI.(13) The emphasis on brain volume as determined by basilar cistern status and presence/degree of midline shift can place heterogeneous injuries within the same Marshall score. Mass lesions are included in the Marshall score, but are treated separately from brain volume status and are further divided by whether surgical intervention was required.
The more recently developed Rotterdam scoring system(14) utilizes some elements of the Marshall score, specifically status of the basilar cisterns and presence/degree of midline shift, along with presence of SAH and IVH. (4, 8–10) This scale differentiates between types of mass lesions, recognizing the more favorable prognosis associated with epidural hematomas (EDH).(8, 12, 15) Although the Marshall CT score has been used in pediatric TBI research(11, 16, 17), neither it nor the Rotterdam CT score have been validated to predict mortality in children.
This study aimed to determine whether the Rotterdam CT score was predictive of in-hospital mortality for children with moderate to severe TBI. We hypothesized that it can be used for mortality risk stratification in children with TBI.
Methods
Patient Selection
Patients were included if they were < 17 years old at admission, had either moderate or severe TBI, and were cared for at Primary Children's Medical Center (PCMC, now called Primary Children's Hospital, PCH) between January, 2002 and December, 2010. For patients prior to 2007, we utilized a previously studied retrospective cohort of 299 infants and children with TBI admitted to PCH (17–19). Moderate TBI was defined as a post-resuscitation Glasgow Coma Score (GCS) of 9 – 12 and severe TBI as a post-resuscitation GCS of 3 – 8. The GCS was assigned by the trauma service in the PCH Emergency Department (ED). Patients were excluded if they died before the initial non-contrast head CT was obtained, if no CT images were obtained at PCH or if the images were not available for review. The primary outcome was in-hospital mortality.
PCH is a freestanding level I trauma center that serves 6 states in the Western region of the United States and had 1200 to 2000 Pediatric Intensive Care Unit (PICU) admissions per year during the study period. This study was approved by the University of Utah Institutional Review Board and was granted a waiver of need for informed consent.
Injury Severity
Injury severity was evaluated using GCS, injury severity score (ISS), mechanism of injury, and use of intracranial pressure (ICP) monitoring. Need for ICP monitoring was determined by the care team independently from this study. Determination of non-accidental trauma (NAT) was made by the hospital's child abuse team.
Assignment of Rotterdam Scores
A pediatric neurosurgeon (JRC) blinded to outcome reviewed the first available non-contrast head CT obtained in the first twenty four hours for each patient and assigned Rotterdam scores according to the rubric reported by Maas et al(14) (Table 1). During the time between the initial analysis of this cohort(18) and the current study, the Radiology Department at PCH migrated imaging platforms and 6 of our patients' CT images were lost and Rotterdam scores could not be assigned.
Table 1.
Rotterdam CT score
| Rotterdam Score Element | Score |
|---|---|
| Basal Cisterns | |
| Normal | 0 |
| Compressed | 1 |
| Absent | 2 |
| Midline Shift | |
| No Shift or shift ≤ 5-mm | 0 |
| Shift > 5-mm | 1 |
| Epidural Mass Lesion | |
| Present | 0 |
| Absent | 1 |
| Intraventricular Blood or tSAH | |
| Absent | 0 |
| Present | 1 |
| Sum Score | +1 |
tSAH = traumatic subarachnoid hemorrhage
Statistical Analysis
Bivariate categorical analyses were performed using the χ2 or Fisher's exact tests, as appropriate given cell size, and the medians of ordinal variables were tested using the Wilcoxon rank sum test.
Prediction models
We first compared pediatric observed mortality to predicted mortality based on the published adult probabilities of death by Rotterdam CT score.(14) We then randomly selected (using “runiform” in Stata) 70% of the PCH patients (prediction cohort) and used logistic regression to derive a mortality prediction equation for the Rotterdam score in children with moderate or severe TBI. This pediatric model was then tested against the remaining 30% of the patients (validation cohort).
As sensitivity analyses, we then derived and validated two separate models, first restricting to children with severe TBI (GCS 3–8), and then excluding children with NAT. Finally, in an exploratory fashion, we examined the effect of adding additional predictors to the pediatric prediction model. Age (years, continuous), GCS, presence of endotracheal tube at time of GCS (yes/no), mechanism of injury (categorical), and injury severity score (ISS) were added sequentially to the prediction model and tested using the likelihood ratio (LR) test. The parameterization (categorical versus continuous) of the Rotterdam score was also tested using the LR test.
All models were assessed for discrimination (the ability of the model to separate patients into distinct categories), calibration (how closely the predicted mortality matches the actual mortality), and overall fit. Model discrimination was evaluated using area under the receiver-operator characteristic curve (AUC, also known as the C-statistic)(20), model calibration was assessed using calibration plots of observed versus predicted mortality and Cox's calibration regression(21), and overall fit was tested using Brier's score.(22) Cox's calibration regression fits the model (true log odds = slope × predicted log odds + intercept) and an ideally calibrated model will have a slope of 1 and an intercept of 0.(21, 23) Brier's score is an overall measure of accuracy originally developed for meteorological forecasting, and a perfectly fit model will have a score of 0.(22) “Chance” predictions of 0.5 for each subject will result in a Brier's score of 0.25. Relevant examples include well-fit neonatal outcome models with Brier's scores of 0.05 or less.(24, 25) We defined statistical significance as p < 0.05 and used Stata version 12 (StataCorp LP, College Station, TX), and the R environment (version 3.0.1) to conduct the analyses. All confidence intervals shown were calculated using exact methods for proportions.
Results
PCH Cohort
Of the 681 patients with moderate to severe TBI identified for inclusion in the PCH cohort, 23 were excluded because they died before the initial head CT was done, 19 were excluded because they did not have a head CT performed at our institution and no images were loaded into our system (post-operative images were not used), 6 were excluded because their CT images were lost during an imaging system migration, and 1 patient was excluded because a name change led to loss of reliable tracking information.
Seventy percent (442/632) of the patients were randomly selected for the prediction cohort and 30% (190/632) for the validation cohort (Table 2). We found no statistically significant differences in mortality, gender, age, injury year, GCS, mechanism of injury, or Rotterdam total score between the prediction and validation cohorts (not shown). Median ISS was higher in the prediction cohort than the validation cohort (25 versus 21, p = 0.02). Overall, 20% (128/632) of the included patients had moderate TBI and 80% (504/632) had severe TBI. In-hospital mortality was 16% (101/632), and 53% of the deaths (54/101) occurred in the first 24 hours.
Table 2.
Demographic, Injury, and CT Characteristics of Children with Moderate to Severe TBI
| Prediction Cohort | Validation Cohort | |||
|---|---|---|---|---|
| Survivors | Non-Survivors | Survivors | Non-Survivors | |
| N = 364 | N = 78 | N= 167 | N = 23 | |
| n(col%) | n(col%) | n(col%) | n(col%) | |
| Gender | ||||
| Male | 227(62) | 38(49) | 106(63) | 19(83) |
| Age | ||||
| Median (IQR) | 6 (2–11) | 5 (1–11) | 6 (2–11) | 6 (1–12) |
| 0–364 days | 32(9) | 18(23) | 17(10) | 5(22) |
| 1 to <5 years | 120(33) | 20(26) | 52(31) | 6(26) |
| 5 to 12 years | 143(39) | 28(36) | 69(41) | 8(35) |
| ≥ 13 years | 69(19) | 12(15) | 29(17) | 4(17) |
| Injury Year | ||||
| 2002–2004 | 107(29) | 18(23) | 46(28) | 7(30) |
| 2005–2006 | 61(17) | 19(24) | 25(15) | 4(17) |
| 2007–2008 | 112(31) | 21(27) | 51(31) | 2(9) |
| 2009–2010 | 84(23) | 20(26) | 45(27) | 10(43) |
| Glasgow Coma Score | ||||
| 3–8 | 286(79) | 74(95) | 122(73) | 22(96) |
| 9–12 | 78(21) | 4(5) | 45(27) | 1(4) |
| Injury Severity Score (ISS) | ||||
| Median (IQR) | 21 (14–29) | 30 (25–38) | 17 (14–25) | 26 (25–35) |
| Mechanism of injury | ||||
| Fall | 75(21) | 6(8) | 39(23) | 2(9) |
| Assault/NAT | 35(10) | 22(28) | 15(9) | 8(35) |
| Bicycle/pedestrian | 71(20) | 18(23) | 40(24) | 2(9) |
| Motor vehicle/ATV | 118(32) | 26(33) | 38(23) | 6(26) |
| Other | 65(18) | 6(8) | 35(21) | 5(22) |
| Basal Cisterns | ||||
| Normal | 305(84) | 15(19) | 143(86) | 9(39) |
| Compressed | 53(15) | 30(38) | 23(14) | 8(35) |
| Absent | 6(2) | 33(42) | 1(1) | 6(26) |
| Midline Shift | ||||
| No shift or shift ≤ 5 mm | 326(90) | 61(78) | 156(93) | 17(74) |
| Shift > 5 mm | 38(10) | 17(22) | 11(7) | 6(26) |
| EpiduralMass Lesion | ||||
| Present | 36(10) | 5(6) | 10(6) | 3(13) |
| Absent | 328(90) | 73(94) | 157(94) | 20(87) |
| IVH or tSAH | ||||
| Absent | 219(60) | 20(26) | 98(59) | 5(22) |
| Present | 145(40) | 58(74) | 69(41) | 18(78) |
| Rotterdam Score | ||||
| 1 | 12(3) | 0(0) | 5(3) | 1(4) |
| 2 | 188(52) | 6(8) | 84(50) | 2(9) |
| 3 | 115(32) | 13(17) | 59(35) | 6(26) |
| 4 | 40(11) | 29(37) | 16(10) | 7(30) |
| 5 | 7(2) | 25(32) | 3(2) | 6(26) |
| 6 | 2(1) | 5(6) | 0(0) | 1(4) |
CT = computed tomography; TBI= traumatic brain injury; col% = column percentage; IQR = Interquartile range;; ISS = injury severity score; NAT = non-accidental trauma; ATV = All-terrain vehicle; IVH = intraventricular hemorrhage; tSAH = traumatic subarachnoid hemorrhage
Non-survivors were more likely to be < 1 year old (32% mortality versus 13–15% in all other age categories, p = 0.001), to have a higher ISS (median 30 versus survivors median 20, p < 0.001), and to have injuries secondary to assault or NAT (38% mortality), bicycle or pedestrian trauma (15%), or motor vehicle/all-terrain vehicle trauma (17%), p < 0.001 across categories. Patients who died were more likely to have severe TBI compared to moderate TBI (19% versus 4%, exact p < 0.001). ICP monitoring devices were placed in 33%, with no difference in monitor placement between the prediction cohort and the validation cohort (35% versus 30%, p = 0.24); among patients with severe TBI, monitor placement occurred in 40% of the prediction cohort and 35% of the validation cohort (p = 0.31).
Not unexpectedly, the 23 children who died before CT images were obtained tended to have more severe TBI (23/23 had a GCS of 3) and overall injuries (median ISS 34 versus 25, p < 0.001) than those included in the study. The 26 children who did not have CT images in our system were similar to the study population in age, gender, GCS, and ISS (p > 0.05).
Rotterdam Score
Rotterdam category 2 was the most common, and this Rotterdam score was found in children with all values of GCS between 3 and 12 (Figure 1). Because this score represents no positive findings, some of those scored as 2 are normal scans. Of the 358 patients with GCS = 3, 140 (39%) had a Rotterdam score of 2. This represents 22% (140/632) of the entire cohort. A score of 1 represents an isolated epidural hematoma without enough mass effect to cause midline shift or compression of the basilar cisterns. All children with a Rotterdam score of 6 had a GCS of 3.
Figure 1. Rotterdam scores and Glasgow Coma Scale (GCS) scores.
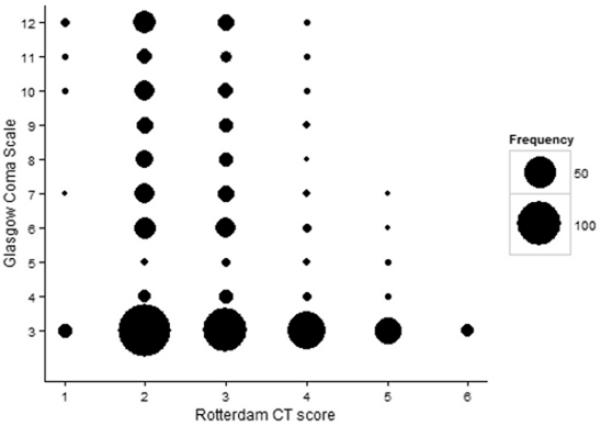
Marker area corresponds to the number of patients at each location in the figure
The Rotterdam score had a direct relationship with mortality in both the adult cohort and the PCH cohort (Table 2, Figure 2). Pediatric mortality was lower than adult mortality for Rotterdam scores of 2 and 3, and higher than adult mortality for scores of 4–6. Children with a Rotterdam score of 1 had a higher mean mortality than adults, but the mortality estimate for category 1 was based on few subjects (n=18) and only one death. Similarly, the pediatric estimate for category 6 was based on only 8 patients, 6 of whom died. We found no secular trend in mortality, as there was no statistically significant linear relationship between mortality and injury year either overall or within any of Rotterdam categories 2 – 5 (not shown).
Figure 2. Observed mortality of adults and children with moderate or severe TBI, by Rotterdam score.
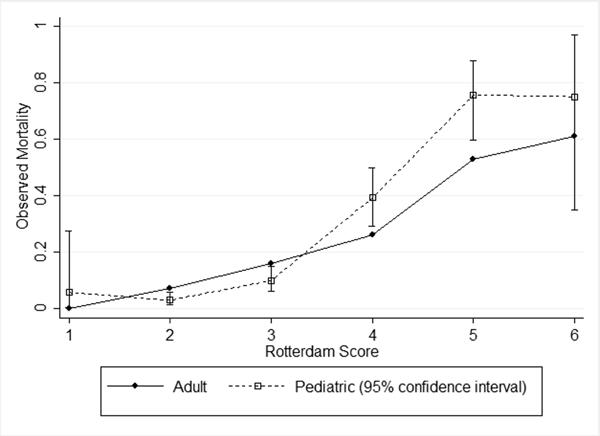
Adult observed mortality is from Maas et al[14], Table 6, pg. 1179
Among children with moderate or severe TBI, the adult probabilities of death by Rotterdam score discriminated mortality in children well(26) (AUC = 0.85, 95% confidence interval [CI] 0.80 – 0.89) (Table 3). However, calibration was fairly poor, as the intercept of the Cox's calibration regression model was significantly different than 0 and the slope was significantly different than 1. Overall fit was also not optimal, as the Brier's score was approximately 0.1. The adult model tended to overestimate pediatric mortality for Rotterdam scores of 2 and 3 and underestimate them for scores of 4 and 5 (Figure 3).
Table 3.
Measures of discrimination, calibration, and overall fit for each Rotterdam score model
| Model | Adult versus Pediatric | New Pediatric Final Model | New Pediatric Model, Severe TBI only | New Pediatric Model, Excluding NAT |
|---|---|---|---|---|
| Population characteristics | GCS 3–12 | GCS 3–12 | GCS 3–8 | GCS 3–12 No NAT |
| Prediction dataset | Maas et al[14] | Prediction Cohort | Prediction Cohort | Prediction Cohort |
| Prediction N | 2,249 | 442 | 360 | 385 |
| Validation dataset | Entire PCH Cohort | Validation Cohort | Validation Cohort | Validation Cohort |
| Validation N | 632 | 190 | 144 | 167 |
| AUC (95% CI) | 0.85 (0.80 – 0.89) | 0.80 (0.68 – 0.91) | 0.81 (0.70 – 0.92) | 0.92 (0.86 – 0.97) |
| Cox's calibration regression | ||||
| Intercept (95% CI) | 1.08 (0.57 – 1.60) | −0.43 (−1.10 – 0.24) | −0.32 (−0.99 – 0.36) | −0.24 (−1.07 – 0.60) |
| Slope (95% CI) | 1.81 (1.46 – 2.15) | 0.86 (0.52 – 1.20) | 0.87 (0.50 – 1.24) | 1.08 (0.63 – 1.52) |
| LRX2 (1) | 165.6 | 31.2 | 28.3 | 43.0 |
| p | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Brier's score | 0.098 | 0.082 | 0.098 | 0.052 |
| Figure | 3 | 4 |
GCS = Glasgow Coma Scale; NAT = Non-accidental Trauma; PCH = Primary Children's Hospital; AUC = Area under the curve; CI = confidence interval; LR = likelihood ratio test
Figure 3. Calibration plot for children with moderate or severe TBI, with predicted mortality from adult Rotterdam score mortality.
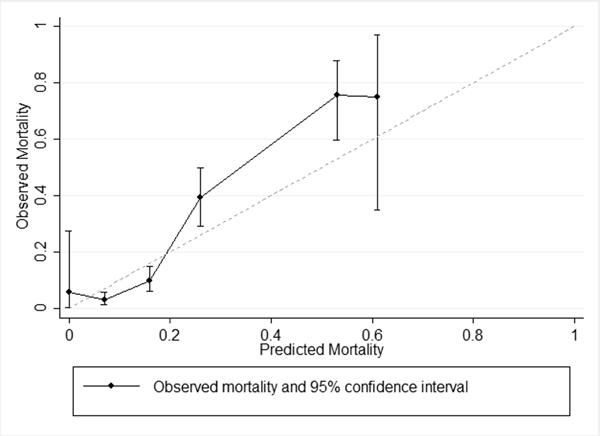
Observed mortality by Rotterdam score (range 1 to 6) for entire PCH cohort. Predicted mortality is derived from Maas et al[14], Table 6, pg. 1179
Because the adult model did not predict mortality well in children, a pediatric-specific model was necessary for more accurate prediction in children. Therefore, we used logistic regression to derive a novel prediction model from the PCH prediction cohort. For children with moderate or severe TBI, predicted mortality = [e−6.57 + (1.527*Rotterdam)]/[1 + e−6.57 + (1.527*Rotterdam)]. This model was then used to compare predicted to actual mortality among subjects in the PCH validation cohort (Table 2). The pediatric Rotterdam model for patients with moderate or severe TBI discriminated mortality in the validation cohort well (AUC = 0.80, 95% CI 0.68 – 0.91) and was acceptably calibrated (Figure 4 and Table 3). The Cox's calibration regression intercept was not different than 0 and the slope was not different than 1, and the overall fit was better than for the adult model (Brier's score = 0.08). Using a categorical variable for the Rotterdam score did not improve prediction over a grouped linear form (LR test p = 0.26), so the simpler model was retained.
Figure 4. Calibration plot for children with moderate or severe TBI, with predicted mortality from new pediatric mortality model.
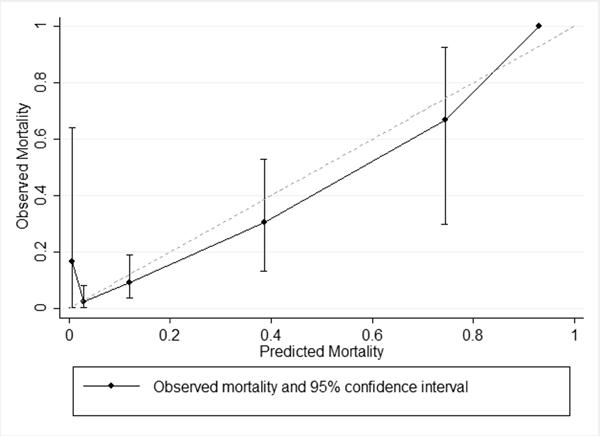
Observed mortality by Rotterdam score (range 1 to 6) for PCH validation cohort. Predicted mortality is from new pediatric mortality model. Category 6 has only one patient in the validation cohort, therefore no 95% confidence interval is available.
In a sensitivity analysis, a separate model restricted to children with severe TBI and tested in the validation cohort's severe TBI patients was not demonstrably better than the model including both moderate and severe TBI patients (Table 3). Another model excluding children with NAT showed excellent predictive performance in that restricted population (Table 3).
Beginning with our overall model for children with moderate or severe TBI, we explored whether additional model parameters improved prediction. Sequential LR tests showed that models including age and the presence of endotracheal tube at the time of GCS were not statistically significantly different than the base model. GCS, mechanism of injury, and ISS were candidates for model inclusion by LR test. A model with GCS alone (Table 4) did not discriminate mortality well, had acceptable calibration, and less than optimal fit. A model with both GCS and the Rotterdam score (Table 4) showed good discrimination, acceptable calibration, and an overall fit quite similar to the Rotterdam-only model. Adding mechanism of injury and ISS to that model (Table 4) led to excellent discrimination, good calibration, and an improved overall fit.
Table 4.
Measures of discrimination, calibration, and overall fit for mortality prediction models in children with moderate or severe TBI
| Model components | Rotterdam alone – Final model | GCS alone | Rotterdam and GCS | Rotterdam, GCS, mechanism, ISS |
|---|---|---|---|---|
| Prediction dataset | Prediction Cohort | Prediction Cohort | Prediction Cohort | Prediction Cohort |
| Prediction N | 442 | 442 | 442 | 442 |
| Validation dataset | Validation Cohort | Validation Cohort | Validation Cohort | Validation Cohort |
| Validation N | 190 | 190 | 190 | 190 |
| AUC (95% CI) | 0.80 (0.68 – 0.91) | 0.70 (0.62 – 0.78) | 0.83 (0.72 – 0.94) | 0.91 (0.84 – 0.98) |
| Cox's calibration regression | ||||
| Intercept (95% CI) | −0.43 (−1.10 – 0.24) | −0.12 (−1.27 – 1.03) | −0.31 (−0.98 – 0.36) | 0.20 (−0.55 – 0.94) |
| Slope (95% CI) | 0.86 (0.52 – 1.20) | 1.13 (0.36 – 1.91) | 0.89 (0.56 – 1.23) | 1.13 (0.72 – 1.53) |
| LRX2 (1) | 31.2 | 15.2 | 37.0 | 59.6 |
| p | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Brier's score | 0.082 | 0.101 | 0.078 | 0.060 |
| Figure | 2 |
TBI = traumatic brain injury; GCS = Glasgow Coma Scale; ISS = Injury Severity Score; AUC = Area under the curve; CI = confidence interval; LR = likelihood ratio test
Discussion
We found that the Rotterdam CT score has a direct relationship with probability of mortality and can be used for mortality risk stratification in children with moderate or severe TBI. Despite accurate discrimination of mortality in children, the prediction model developed using adult TBI data overestimated mortality in children with lower Rotterdam scores and underestimated mortality among those with higher scores. Our novel pediatric prediction model shows good discrimination, calibration, and overall fit. It has now been validated using a large pediatric TBI cohort and can be used in pediatric studies as one measure of TBI severity.
The limitations of the GCS as a measure of TBI severity are well-described(27), and imaging data have been identified as a priority to include in new severity measures.(28) TBI is heterogeneous(8), which complicates CT classification and mortality risk estimation. CT scoring systems in general, and in particular the Rotterdam score, attempt to account for the effects of injury heterogeneity. This approach allows for better discrimination and mortality prediction in adults.(13, 14) Maas et al included adults with moderate TBI in their Rotterdam derivation, and we found the same low rate of mortality in children with moderate TBI. These children may suffer important morbidity, however. Restricting our model to children with severe TBI did not improve mortality prediction and limited generalizability; we therefore included children with moderate or severe TBI in our final model. The variability of GCS values within each Rotterdam score and the variability of Rotterdam values within each GCS category are consistent with brain injury heterogeneity and the sensitivity of GCS to obfuscating factors such as sedation and neuromuscular blockade.
We found it interesting that the new pediatric model, despite its simplicity, was robust; mortality prediction did not improve substantially until three additional variables were added, one of which (ISS) is only rarely available to the clinician early in the treatment course. A limitation is that pupillary reactivity, one of the strongest predictors of outcome in adults with TBI, was not present in our dataset.
Potential mechanisms to explain the differences between the Rotterdam score's more linear relationship with adult mortality and more logistic relationship with pediatric mortality will bear further study.
This paper is the first to validate hospital mortality prediction using the Rotterdam CT scores in a pediatric TBI population. We found a mortality rate of 19% among children with severe TBI from a single center over nine years, similar to a large multi-center trial in pediatric TBI whose overall mortality was 16% (29) and several other single-center analyses of severe TBI with mortality rates ranging from 14–22% (16, 30, 31). Inclusion criteria for these studies did vary somewhat; the youngest patients were as young as one month(31) or as old as one year(29) and one study required an acute brain injury on CT(29) while others did not(30, 31). Two studies of head injury in children, a recent large, multi-center cohort study from Tasker et al, and a single-center study from Hirsch et al reported much lower mortality of 9% and 5%, respectively(8, 32); however, the selection criteria used to identify cases in both studies likely led to the inclusion of some children with mild TBI. A limitation to our study and many of the above is that some children with fairly mild head injury may have been categorized as “severe” due to a low presenting GCS because of sedation or neuromuscular blockade. Nearly one-quarter of our cohort (22%) had a GCS of 3 and a Rotterdam score of 2, which may represent a CT without positive findings.
Strengths of this analysis include use of one of the largest reported cohorts of pediatric patients with moderate to severe TBI. We did not find a secular trend in mortality in our cohort, but we limited the impact of potential improvements in clinical care on our results by randomly selecting prediction and validation cohorts from the entire time span. Like most mortality risk models(33), ours may need to be re-calibrated in the future if outcomes change. Receiver-operator analysis, Cox's calibration regression, and the Brier's score are well-developed tools to evaluate prediction models.
Our study has several other limitations. We studied a cohort of 632 children with moderate to severe TBI which, while large for pediatric studies, is smaller than the patient population in either of the original adult reports.(13, 14) We had small sample sizes in Rotterdam categories 1 and 6, and the mortality point estimates for those categories should be used conservatively. The oldest data in the cohort are now more than ten years old, and the care of children with TBI may have improved since then. The retrospective design dictated what information was available for review and how the Rotterdam scores could be assigned. Although data collection for all presented information was complete, the information available was limited to what was documented during hospitalization. For example, although we were able to collect use of ICP monitoring as an indicator of injury severity, it was beyond the scope of this study to comment on the presence or severity of intracranial hypertension. Some children may not have been deemed salvageable and may not have been provided an ICP monitor.(3, 19) Information on cause of death and the prevalence of brain death versus care limitation or withdrawal is not available in the dataset.
The Rotterdam CT scores for the PCH cohort were assigned by a single neurosurgeon blinded to patient outcome. Chun et al reported that neuroradiologists and neurosurgeons were consistent and accurate when assigning both Marshall and Rotterdam head injury characteristics with acceptable agreement (average Bland-Altman coefficients of variation of 12.7% and 21.9%, respectively).(34)
In conclusion, children with TBI have better survival than adults in Rotterdam CT score categories representing less severe injuries, and worse survival than adults in higher score categories. Using adult probabilities of mortality overestimated pediatric mortality in children with low scores and underestimated mortality in those with high scores. A novel, validated pediatric mortality model based on the Rotterdam score is accurate in children with moderate or severe TBI and can be used for mortality risk stratification in clinical care and future studies.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Author Contributions KL, SB, KSB, JRC, and TB designed the study, KL, HT, and RM obtained the PCH data through chart review and trauma registry query, JRC assigned the Rotterdam scores, KL, SB, and TB analyzed the data, KL wrote the first draft of the manuscript, and all authors contributed to its revision. TB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have seen and approved this final version of the manuscript.
References
- 1.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 3. Prehospital airway management. Pediatr Crit Care Med. 2003;4(3 Suppl):S9–11. [PubMed] [Google Scholar]
- 2.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 3.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 4.Fearnside MR, Cook RJ, McDougall P, et al. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7(3):267–279. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 5.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasaki O, Shiozaki T, Hamasaki T, et al. Prognostic Indicators and Outcome Prediction Model for Severe Traumatic Brain Injury. The Journal of Trauma. 2009:304. doi: 10.1097/TA.0b013e31815d9d3f. [DOI] [PubMed] [Google Scholar]
- 7.Ong L, Selladurai BM, Dhillon MK, et al. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24(6):285–291. doi: 10.1159/000121057. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch W, Schobess A, Eichler G, et al. Severe head trauma in children: cranial computer tomography and clinical consequences. Paediatr Anaesth. 2002;12(4):337–344. doi: 10.1046/j.1460-9592.2002.00837.x. [DOI] [PubMed] [Google Scholar]
- 9.Corral L, Herrero J, Monfort J, et al. First CT findings and improvement in GOS and GOSE scores 6 and 12 months after severe traumatic brain injury. Brain Injury. 2009:403–410. doi: 10.1080/02699050902788477. [DOI] [PubMed] [Google Scholar]
- 10.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 11.Levin H, Aldrich E, Saydjari C, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992:435. doi: 10.1227/00006123-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lobato RD, Cordobes F, Rivas JJ, et al. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg. 1983;59(5):762–774. doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 13.Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computed tomography. J Neurosurg. 1991;75(1S):14–20. [Google Scholar]
- 14.Maas AI, Hukkelhoven CW, Marshall LF, et al. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. discussion 1173–1182. [DOI] [PubMed] [Google Scholar]
- 15.The Brain Trauma Foundation Guidelines: Computed tomography scan features. J Neurotrauma. 2000;17(6–7):597–627. doi: 10.1089/neu.2000.17.597. [DOI] [PubMed] [Google Scholar]
- 16.Claret Teruel G, Palomeque Rico A, Cambra Lasaosa FJ, et al. Severe head injury among children: computed tomography evaluation as a prognostic factor. J Pediatr Surg. 2007;42(11):1903–1906. doi: 10.1016/j.jpedsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Liesemer K, Bratton SL, Zebrack CM, et al. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma. 2011;28(5):755–762. doi: 10.1089/neu.2010.1518. [DOI] [PubMed] [Google Scholar]
- 18.Zebrack M, Dandoy C, Hansen K, et al. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56–64. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 19.Bailey BM, Liesemer K, Statler KD, et al. Monitoring and prediction of intracranial hypertension in pediatric traumatic brain injury: clinical factors and initial head computed tomography. J Trauma Acute Care Surg. 2012;72(1):263–270. doi: 10.1097/TA.0b013e31822a9512. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed John Wiley and Sons, Inc.; 2000. [Google Scholar]
- 21.Cox DR. Two further applications of a model for binary regression. Biometrika. 1958;45(3/4):562–565. [Google Scholar]
- 22.Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev. 1950;78(1):1–3. [Google Scholar]
- 23.Hyam JA, Welch CA, Harrison DA, et al. Case mix, outcomes and comparison of risk prediction models for admissions to adult, general and specialist critical care units for head injury: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl 2):S2. doi: 10.1186/cc5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manktelow BN, Draper ES, Field DJ. Predicting neonatal mortality among very preterm infants: a comparison of three versions of the CRIB score. Archives of disease in childhood Fetal and neonatal edition. 2010;95(1):F9–F13. doi: 10.1136/adc.2008.148015. [DOI] [PubMed] [Google Scholar]
- 25.Manktelow BN, Seaton SE, Field DJ, et al. Population-based estimates of in-unit survival for very preterm infants. Pediatrics. 2013;131(2):e425–432. doi: 10.1542/peds.2012-2189. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr., Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–2546. [PubMed] [Google Scholar]
- 27.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manley GT, Maas AI. Traumatic brain injury: an international knowledge-based approach. JAMA. 2013;310(5):473–474. doi: 10.1001/jama.2013.169158. [DOI] [PubMed] [Google Scholar]
- 29.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 30.Coates BM, Vavilala MS, Mack CD, et al. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit Care Med. 2005;33(11):2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducrocq SC, Meyer PG, Orliaguet GA, et al. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–467. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 32.Tasker RC, Fleming TJ, Young AE, et al. Severe head injury in children: intensive care unit activity and mortality in England and Wales. Br J Neurosurg. 2011;25(1):68–77. doi: 10.3109/02688697.2010.538770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. 2013;14(7):673–681. doi: 10.1097/PCC.0b013e31829760cf. [DOI] [PubMed] [Google Scholar]
- 34.Chun KA, Manley GT, Stiver SI, et al. Interobserver variability in the assessment of CT imaging features of traumatic brain injury. J Neurotrauma. 2010;27(2):325–330. doi: 10.1089/neu.2009.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]


