Figure 1.
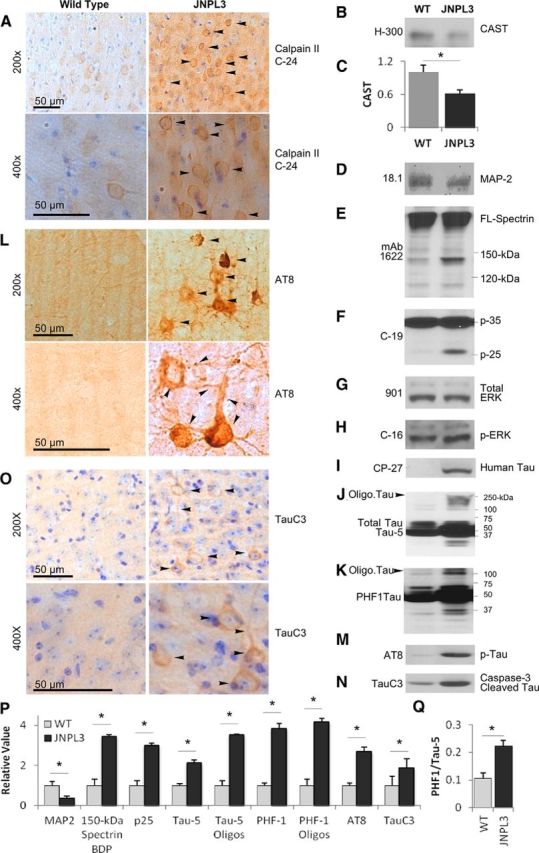
Cal II hyperactivation decreases CAST and promotes cytoskeletal protein breakdown in JNPL3 brains. Brain sections from female mice were immunostained with cal II antibody (C-24, A), AT8 (L), TauC3 (O), and images were captured from the cortex at 200× and 400× magnification. Arrowheads indicate increased cal II, AT8, and TauC3 staining in JNPL3 mice compared with wild type mice (A, L, O). Calpain substrates CAST (B, C, n = 7–9, p < 0.05) and MAP2 (D, P, p < 0.05) are reduced, 150 kDa spectrin breakdown product (150 kDa Spectrin BDP; E, P, p < 0.05), and conversion of cdk5 subunit p35 to p25 are increased (F, P, p < 0.05), whereas activated pErk (H) and total Erk (G) is unaffected in JNPL3 mouse brains. Human tau is present only in JNPL3 mouse brains (I); total tau levels are increased (Tau-5; J, P, p < 0.05). Tau hyperphosphorylation is evidenced by robust AT8-positive neurons compared with WT mice (see arrowheads in L), increased PHF1 (K, P, p < 0.05), and the ratio of PHF1 to total tau (Q, p < 0.05) and AT8 (M, P, p < 0.05) epitopes on immunoblots in JNPL3 mice. Increased TauC3 (N, P, p < 0.05; O) and oligomeric tau (see arrowheads in J, K; see P for quantification of Tau-5 and PHF1 oligomers, p < 0.05) are also observed in JNPL3 mouse brains. Late-stage diseased JNPL3 mice (35 months, n = 4 unless otherwise indicated) along with age-matched WT mice were used in A–O. Scale bar, 50 μm. Oligo. Tau, Oligomeric Tau; Tau-5 oligos, Tau-5 oligomers; PHF1 oligos, PHF1 oligomers. All the quantification data in C, P, and Q are from 17-month-old animals. Error bars indicate SEM, *p < 0.05, C, P, and Q measured by Student's t test.
