Abstract
AIM: To analyze the DNA copy number of target genes NF2, TIMP3, ST13, TOB2, BIK, and TP and the reference microsatellite markers D22S283, D22S423, and D22S274 mapped on 22q12-qter in liver fluke related cholangiocarcinoma (CCA) and define its correlation with clinical parameters.
METHODS: Quantitative real time PCR (qPCR) was used for determining allelic imbalances in 65 liver fluke related CCA tissues. Statistical correlations between allelic imbalances and clinicopathological parameters, i.e. age, sex, tumor stage, histological type, blood vessel invasion, nerve invasion and lymphatic invasion were evaluated by means of the χ2 test. Cox regression analysis was used for determining patient’s survival.
RESULTS: Amplifications of the TP (22q13.33), TOB2 (22q13.2-13.31), D22S283 (22q12.3), TIMP3 (22q12.3) and NF2 (22q12.2) were found in 35 (53.8%), 28 (43.1%), 27 (41.5%), 24 (36.9%), and 24 (36.9%), respectively. Losses at the D22S423 (22q13.1-13.2) and BIK (22q13.31) were detected in 26 (40%) and 23 (35.4%), respectively. Significant correlations were observed between lymphatic invasion and allelic losses of BIK (P = 0.025) and D22S283 (P = 0.041). Univariate and multivariate Cox regression analysis revealed D22S283 amplification as an independent predictor of good prognosis (P = 0.006, death hazard ratio = 0.411, 95% CI = 0.217-0.779) and blood vessel invasion as an independent poor prognostic factor (P = 0.042, death hazard ratio = 1.911, 95% CI = 1.022-3.571) in CCA patients.
CONCLUSION: This study provides evidence for the involvement of gene amplification and deletion on chromosome 22q in liver fluke related CCA. This is the first report of D22S283 amplification as an independent indicator of favorable prognosis in liver fluke related CCA.
Keywords: Liver fluke related cholangiocarcinoma, Chromosome 22q, D22S283, Allelic imbalance, Quantitative real time PCR
INTRODUCTION
Cholangiocarcinoma (CCA) which arises from bile duct epithelium is a common hepatobiliary malignancy found in Northeastern Thais. Liver fluke (Opisthorchis viverrini) infection related to cholangiocarcinogenesis is strongly supported by evidence from both experimental and epidemiological studies[1,2]. In addition, exogenous and/or endogenous N-nitroso compounds have also been claimed to be responsible[1,2]. These studies suggest that liver fluke infection causes chronic inflammatory reactions and enhance the susceptibility of bile duct epithelium to carcinogenic chemicals such as N-nitroso compounds leading to genetic and epigenetic damages in the cells. CCA accounts for about 89% of all liver cancer cases in Khon Kaen with the highest incidence in the world (97.4/105 in males and 39.0/105 in females)[3]. Khon Kaen is one of the largest provinces in Northeast Thailand where the liver fluke is highly endemic. Most patients are diagnosed at late stage and difficult to cure successfully because of advanced metastasis at the time of diagnosis. The 3-year survival rates are 33%, 30% and 12% for stage III, stage IVa, and stage IVb, respectively, whereas 5-year survival rates are 0% for all three late stages[4]. CCA, like other common epithelial cancers is believed to develop through a multistep process. However, the molecular mechanism of carcinogenesis of CCA remain unclear. A series of different genes and chromosomal regions may be deleted or amplified in the tumor genome. Several genes are reported to be involved in CCA, e.g. p53 and MDM2[5], p16[6], K-ras[6], hMLH1 and hMSH2[7,8], and COX-2[9,10]. At the chromosomal levels, changes of several chromosomal arms have been reported, e.g. 13q[11], 6q, 9p and 17p[12], 17q, 5p, 6q, and 18q[13].
There are reports of genetic changes on the chro-mosomal region 22q in other epithelial cancers such as colorectal cancer[14,15], oral cancer[16], breast cancer[17], gastric carcinoma[18] and ovarian carcinoma[19] , except CCA. Alteration in gene copy number by amplification or deletion is a common mechanism that leads to deregulation of gene expression and finally to neoplastic transformation. Investigation of the prognostic or predictive significances of these genetic alterations requires a reliable and sensitive method for the measurement of gene copy number in clinical tumor samples. Quantitative real time PCR is increasingly used to quantify copy numbers of nucleic acids in clinical applications[20]. The measurement of gene copy number by qPCR has frequently been reported for human tumors including stomach cancer[21], neuroblastoma[22], and oligodendroglioma[23]. Our comparative genomic hybridization data in CCA showed copy number alteration at 22q13 at 21%. Taken together, this study aimed to investigate the allelic imbalance of the chromosomal region 22q12-qter by means of SYBR Green I-based qPCR assay and define its candidate genes which may be involved in carcinogenesis and pathogenesis of liver fluke related CCA. We also correlated our findings with clinical parameters including patient survival.
MATERIALS AND METHODS
Samples and DNA extraction
Sixty-five samples were obtained from liver fluke related CCA patients undergoing surgery at Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand. The patients’ data and survival time are shown in Table 1. Informed consent was obtained from each patient prior to sampling under the guideline approved by the Ethical Committee of Khon Kaen University. Tumor tissues containing 70% or more tumor cells were prepared for DNA extraction following standard method. Peripheral blood samples collected from 50 healthy blood donors were used to extract DNA which was then pooled to yield 5 pooled DNA samples (10 cases each) for normal DNA copy number determination. DNA extracted from normal placenta was used for setting a standard curve.
Table 1.
Patients’ data including survival time
| No. | Age(yr) | Sex | Staging | Histological grading |
Invasion |
Survival time (wk) | ||
| Vessel | Nerve | Lymph | ||||||
| 1 | 61 | Female | IVa | Adenosquamous | Yes | No | Yes | 16.85 |
| 2 | 50 | Male | IVb | Well differentiated | Yes | Yes | Yes | 27.28 |
| 3 | 56 | Male | IVb | Poorly differentiated | Yes | No | Yes | 4.85 |
| 4 | 54 | Male | IVa | Well differentiated | Yes | No | No | 56.57 |
| 5 | 46 | Male | IVb | Adenosquamous | Yes | No | Yes | 7.71 |
| 6 | 53 | Male | IVa | Well differentiated | Yes | No | Yes | 42.14 |
| 7 | 64 | Male | IVa | Poorly differentiated | Yes | No | Yes | 7.28 |
| 8 | 66 | Male | IVb | Poorly differentiated | Yes | No | Yes | 7.00 |
| 9 | 72 | Male | IVb | Unclassified | No | No | Yes | 20.14 |
| 10 | 64 | Female | IVb | Unclassified | Yes | Yes | Yes | 7.14 |
| 11 | 49 | Male | IVb | Well differentiated | Yes | No | No | 15.57 |
| 12 | 53 | Male | III | Poorly differentiated | Yes | No | Yes | 242.85 |
| 13 | 53 | Female | IVa | Well differentiated | Yes | No | No | 2.00 |
| 14 | 62 | Male | IVb | Adenosquamous | Yes | Yes | Yes | 3.00 |
| 15 | 56 | Male | II | Poorly differentiated | Yes | No | Yes | 30.71 |
| 16 | 55 | Male | IVb | Moderately differentiated | Yes | No | Yes | 72.57 |
| 17 | 68 | Male | IVb | Moderately differentiated | Yes | Yes | Yes | 32.00 |
| 18 | 72 | Female | IVb | Poorly differentiated | Yes | No | No | 18.57 |
| 19 | 65 | Female | IVb | Well differentiated | Yes | Yes | Yes | 20.85 |
| 20 | 52 | Male | IVa | Moderately differentiated | Yes | Yes | Yes | 36.71 |
| 21 | 53 | Male | IVb | Unclassified | Yes | No | No | 27.14 |
| 22 | 66 | Male | IVb | Moderately differentiated | Yes | Yes | Yes | 10.42 |
| 23 | 49 | Male | IVb | Well differentiated | No | Yes | Yes | 67.71 |
| 24 | 56 | Male | IVb | Adenosquamous | Yes | No | Yes | 10.00 |
| 25 | 41 | Male | IVb | Moderately differentiated | No | Yes | No | 47.14 |
| 26 | 67 | Female | IVb | Poorly differentiated | No | No | Yes | 118.28 |
| 27 | 63 | Male | IVa | Unclassified | Yes | Yes | Yes | 73.85 |
| 28 | 39 | Female | IVa | Well differentiated | Yes | Yes | Yes | 30.42 |
| 29 | 44 | Male | IVb | Unclassified | Yes | Yes | Yes | 18.71 |
| 30 | 60 | Female | IVb | Poorly differentiated | Yes | No | Yes | 75.71 |
| 31 | 40 | Male | III | Poorly differentiated | Yes | No | Yes | 26.57 |
| 32 | 62 | Male | IVb | Unclassified | No | No | Yes | 40.00 |
| 33 | 43 | Female | IVb | Moderately differentiated | No | Yes | Yes | 29.42 |
| 34 | 70 | Male | IVa | Unclassified | Yes | No | Yes | 79.42 |
| 35 | 63 | Female | III | Unclassified | No | No | No | 80.71 |
| 36 | 61 | Male | IVb | Moderately differentiated | No | Yes | Yes | 77.28 |
| 37 | 48 | Male | III | Unclassified | No | No | No | 94.00 |
| 38 | 55 | Male | IVb | Moderately differentiated | Yes | Yes | Yes | 12.14 |
| 39 | 50 | Female | IVb | Moderately differentiated | No | Yes | Yes | 40.14 |
| 40 | 39 | Male | IVb | Adenosquamous | Yes | No | Yes | 9.14 |
| 41 | 58 | Male | IVb | Well differentiated | Yes | Yes | Yes | 70.42 |
| 42 | 40 | Female | III | Moderately differentiated | No | Yes | No | 17.42 |
| 43 | 36 | Female | IVb | Well differentiated | No | Yes | Yes | 35.14 |
| 44 | 75 | Male | IVb | Poorly differentiated | Yes | No | Yes | 22.14 |
| 45 | 40 | Male | III | Well differentiated | No | Yes | Yes | 3.42 |
| 46 | 52 | Male | IVa | Adenosquamous | No | Yes | Yes | 49.00 |
| 47 | 49 | Male | IVb | Moderately differentiated | No | Yes | Yes | 27.42 |
| 48 | 63 | Male | IVb | Well differentiated | Yes | Yes | Yes | 18.28 |
| 49 | 50 | Male | IVb | Adenosquamous | Yes | Yes | Yes | 35.00 |
| 50 | 56 | Male | III | Poorly differentiated | Yes | No | Yes | 41.14 |
| 51 | 61 | Male | IVb | Poorly differentiated | Yes | No | No | 3.00 |
| 52 | 58 | Male | IVb | Well differentiated | Yes | No | Yes | 13.00 |
| 53 | 55 | Female | IVb | Well differentiated | Yes | Yes | Yes | 67.85 |
| 54 | 55 | Female | IVb | Poorly differentiated | No | Yes | Yes | 40.85 |
| 55 | 50 | Male | nd | Well differentiated | Yes | No | Yes | 10.71 |
| 56 | 38 | Male | IVb | Well differentiated | Yes | Yes | Yes | 27.85 |
| 57 | 46 | Female | nd | Well differentiated | Yes | Yes | Yes | 9.71 |
| 58 | 43 | Male | IVb | Well differentiated | Yes | No | Yes | 65.00 |
| 59 | 54 | Male | IVa | Well differentiated | No | No | No | 9.14 |
| 60 | 42 | Male | IVb | Moderately differentiated | No | Yes | Yes | 7.14 |
| 61 | 34 | Male | IVa | Well differentiated | Yes | Yes | Yes | 9.00 |
| 62 | 61 | Female | IVa | Unclassified | No | No | Yes | 89.14 |
| 63 | 62 | Female | IVb | Poorly differentiated | Yes | Yes | Yes | 45.28 |
| 64 | 51 | Male | IVa | Well differentiated | Yes | Yes | Yes | 55.42 |
| 65 | 46 | Male | IVa | Poorly differentiated | Yes | Yes | Yes | 18.71 |
Polymorphic markers and gene specific primers
Six target genes and 3 reference microsatellite markers spanning from centromere to telomere of the chromosomal region 22q12-qter were selected for DNA copy number quantification. The genes were NF2 (Neurofibromin 2), TIMP3 (Tissue Inhibitor of Metalloproteinase 3), D22S283, D22S423, ST13 (Suppression of Tumorigenicity 13), TOB2 (Transducer of ERBB2, 2), BIK (BCL2-interacting Killer), D22S274, and TP (Thymidine Phosphorylase). The sequence and location of each locus are shown in Table 2.
Table 2.
Sequences of markers located at chromosomal region 22q12-qter including two housekeeping genes
| Gene/ marker | Band | Primer sequence (5’-3’) |
| D22S283 | 22q12.3 | 5’ ACC AAC CAG CAT CAT CAT 3’ 5’ AGC TCG GGA CTT TCT GAG 3’ |
| D22S423 | 22q13.1-13.2 | 5’ TGC AAA CTC AGC CTG GA 3’ 5’ ACC AAC TGA CTC GTT TAG GTC AT 3’ |
| D22S274 | 22q13.3 | 5’ GTC CAG GAG GTT GAT GC 3’ 5’ AGT GCC CAT TTC TCA AAA TA 3’ |
| NF2 | 22q12.2 | 5’ AAG AGC AAG CAT CTG CAG GA 3’ 5’ TGG TAT TGT GCT TGC TGC TG 3’ |
| TIMP3 | 22q12.3 | 5’ TGT CTC TGG ACC GAC ATG CT 3’ 5’ TGG CGC TCA GGG ATC TGT G 3’ |
| ST13 | 22q13.2 | 5’ GTT ACA CTA TTT AAG AGC TGA AT 3’ 5’ GGT CTT CTA CTT AGA AAA ACC TA 3’ |
| TOB2 | 22q13.2-q13.31 | 5’ GAA GAC ACC CCT TTG TGG AA 3’ 5’ TCT GTG GTC TTG GGT GCT C 3’ |
| BIK | 22q13.31 | 5’ ATG ACC ACT GCC CTG GAG 3’ 5’ CTA AAC ACA GGC CAC AGT TAA CC 3’ |
| TP | 22q13.33 | 5’ GGG GCT CAA GTC GCG AGG 3’ 5’ CCT GCG GGG ATG CCT GAC 3’ |
| β-actin (Reference) | 7p15-p12 | 5’ TCA CCC ACA CTG TGC CCA TCT ACG A 3’ 5’ CAG CGG AAC CGC TCA TTG CCA ATG G 3’ |
| GAPDH (Reference) | 12p13 | 5’ ACA GTC CAT GCC ATC ACT GCC 3’ 5’ GCC TGC TTC ACC ACC TTC TTG 3’ |
Quantitative real time PCR system using a standard curve method
Allelic imbalance on the chromosomal region 22q12-qter was analyzed by SYBR Green I based qPCR using Rotor-Gene 2000 Real Time Amplification System (Corbett Research, Australia). Since SYBR Green I dye is a nonspecific dsDNA intercalating dye, we made the reaction specific by analyzing the annealing and signal acquisition temperatures for each primer. A relative standard curve was constructed for each locus using a 2-fold serial dilution of human placental DNA to get 4 different concentrations. Placental DNA with known concentration was used as a control to validate experimental precision. A relative DNA copy number was calculated for each sample as a ratio of DNA copy number for target locus and the average DNA copy number for 2 reference loci GAPDH and β-actin housekeeping genes. Relative DNA copy numbers of 9 tested loci in 5 pooled normal leukocyte DNA (n = 45) were calculated for normal reference range. The relative DNA copy number in the sample was interpreted as loss or gain when the ratio was less than mean - 2SD or more than mean + 2SD of normal reference range, respectively.
Quantitative PCR was conducted in triplicate in a 20 μL reaction volume containing 67 mmol/L Tris Base, 16.6 mmol/L ammonium sulfate in a 10 mL/L Tween 20, 3 mmol/L MgCl2, 5 pmol each primer, 100 μmol/L each deoxynucleoside triphosphate (dNTP), 1X SYBR Green I and 1 unit Taq polymerase with different concentration of genomic DNA. Each locus was amplified using optimal conditions. The thermal cycling conditions comprised 94°C for 300 s, and 40 cycles of 94°C for 15 s, 46°C -58°C for 20 s and 72°C for 15 s and hold at 72°C for 600 s. To assure the reproducibility and accuracy of qPCR, experiments were repeated when a coefficient of variation for triplicate samples was higher than 10% or a PCR efficiency lower than 0.85 was observed.
Statistical analysis
The association between allelic imbalance and clinical features was analyzed by χ2 test using SPSS statistical software version 10.0 for Windows (SPSS Inc, Chicago, Ill). Survival curves were analyzed by Kaplan-Meier and the significant difference confirmed by Log rank test. Univariate and multivariate Cox regression models were also used for survival analysis. P < 0.05 was defined as significance.
RESULTS
Allelic imbalance of 9 target loci on chromosome 22q12-qter
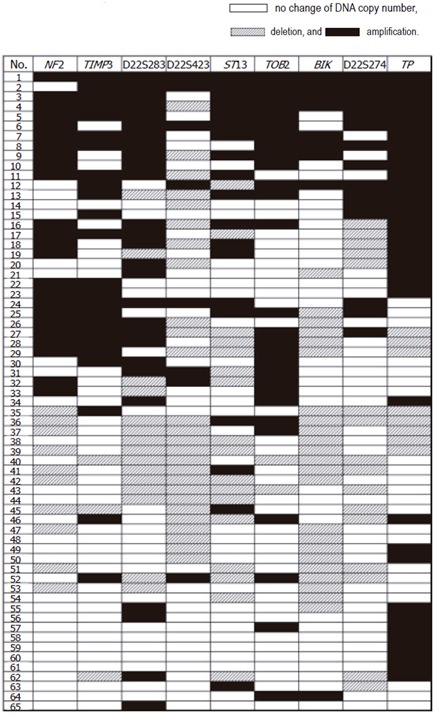
Sixty-five samples from liver fluke related CCA patients were investigated for aberrations in DNA copy number of 9 target genes on 22q12-qter. The normal reference range statistically calculated from 5 pooled normal leukocyte DNA was 0.82-1.33, which was derived from 1.08 ± 0.13 (mean ± 2SD; n = 45). Amplification frequencies higher than 30% were observed in TP (53.8%), TOB2 (43.1%), D22S283 (41.5 %), TIMP3 (36.9%), and NF2 (36.9%). Loss frequencies more than 30% were detected in D22S423 (40%) and BIK (35.4%). Allelic imbalance of each locus is shown in Figure 1. Fine mapping of chromosomal region 22q12-qter from centromeric (NF2) to telomeric ends (TP) in CCA is shown in Figure 2. The regions of common amplification were observed at TP, TOB2 and the 6.6 cM region between NF2 and D22S283. The regions of common loss were D22S423 and BIK.
Figure 1.

Percentages of deletion and amplification in each locus.
Figure 2.
Fine mapping of chromosomal region 22q12-qter in CCA from centromeric (NF2) to telomeric (TP) ends. The regions of common amplification are TP, TOB2, and between NF2 and D22S283. The common deleted regions are D22S423 and BIK.
Correlation of clinicopathological features and allelic imbalance on chromosome 22q12-qter
Associations between allelic imbalances of 9 target loci and clinicopathological parameters, i.e. age, sex, tumor stage, histological type, blood vessel invasion, nerve invasion and lymphatic invasion were evaluated by means of the χ2 test. The mean age of the 65 patients was 54 years. Lymphatic invasion was observed in patients with deletion at D22S283 (P = 0.041) and BIK (P = 0.025). Using Kaplan-Meier survival analysis, the median survival time was 30.42 weeks (SE = 4.29, 95% CI = 22.00-38.84), while the 3-yr and 5-yr survival rate were 9.2 % and 1.5 %, respectively. Cox regression analysis by univariate model showed poor outcome for patients with blood vessel invasion (P = 0.021, death hazard ratio = 2.080, 95% CI = 1.120-3.877) but improved survival with D22S283 amplification (P = 0.003, death hazard ratio = 0.384, 95 % CI = 0.202-0.728) (Table 3). Parameters with P < 0.1 in the univariate analysis were included in the multivariate model of Cox regression analysis for survival. The multivariate analysis revealed the significance of D22S283 amplification as an independent predictor of better prognosis compared with those without (P = 0.006, death hazard ratio = 0.411, 95% CI = 0.217-0.779), whereas blood vessel invasion was revealed as an independent poorer prognostic factor in CCA patients (P = 0.042, death hazard ratio = 1.911, 95% CI = 1.022-3.571) as shown in Table 3. Survival prediction by multivariate Cox regression analysis of D22S283 and blood vessel invasion is shown in Figure 3.
Table 3.
Cox regression analysis using univariate and multivariate models in CCA patients
| Variables | Categories |
Univariate analysis |
Multivariate analysis |
||
| P-value | HR (95% CI) | P-value | HR (95% CI) | ||
| Stage | IVa + IVb vs II + III | NS | 1.560 (0.660-3.690) | ||
| Histology | Poorly vs Unclassified | NS | 1.152 (0.507-2.619) | ||
| Moderate vs Unclassified | NS | 0.994 (0.473-2.087) | |||
| Well diff vs Unclassified | NS | 0.929 (0.429-2.013) | |||
| Vessel invasion | Yes vs No | 0.021 | 2.080 (1.120-3.877) | 0.042 | 1.911 (1.022-3.571) |
| Nerve invasion | Yes vs No | NS | 1.328 (0.770-2.293) | ||
| Lymphatic invasion | Yes vs No | NS | 1.591 (0.716-3.536) | ||
| TP | Deletion vs Normal | NS | 0.791 (0.314-1.997) | ||
| Amplification vs Normal | NS | 1.034 (0.576-1.857) | |||
| D22S274 | Deletion vs Normal | NS | 1.186 (0.625-2.250) | ||
| Amplification vs Normal | NS | 0.869 (0.439-1.718) | |||
| BIK | Deletion vs Normal | NS | 1.271 (0.704-2.294) | ||
| Amplification vs Normal | NS | 1.714 (0.771-3.810) | |||
| TOB2 | Deletion vs Normal | NS | 1.176 (0.277-4.995) | ||
| Amplification vs Normal | NS | 0.945 (0.545-1.641) | |||
| ST13 | Deletion vs Normal | NS | 0.913 (0.466-1.788) | ||
| Amplification vs Normal | NS | 0.938 (0.500-1.761) | |||
| D22S423 | Deletion vs Normal | NS | 1.183 (0.667-2.097) | ||
| Amplification vs Normal | NS | 0.896 (0.366-2.192) | |||
| D22S283 | Deletion vs Normal | NS | 0.793 (0.397-1.585) | ||
| Amplification vs Normal | 0.003 | 0.384 (0.202-0.728) | 0.006 | 0.411 (0.217-0.779 ) | |
| TIMP3 | Deletion vs Normal | NS | 1.758 (0.527-5.864) | ||
| Amplification vs Normal | NS | 0.736 (0.418-1.296) | |||
| NF2 | Deletion vs Normal | NS | 0.929 (0.420-2.055) | ||
| Amplification vs Normal | NS | 0.621 (0.344-1.122) | |||
Figure 3.

Survival curve by multivariate Cox regression analysis. A: Multivariate Cox regression analysis revealed D22S283 copy number amplification as an indicator of favorable prognosis; B: blood vessel invasion showed poor prognosis in liver fluke related CCA patients.
DISCUSSION
Gene deletion and amplification are common events in tumorigenesis and progression. In this study, we examined the allelic imbalance of chromosomal region 22q12-qter by SYBR Green I-based qPCR assay and determined its association with clinicopathological parameters and survival in 65 CCA patients. TP showed the highest frequency of genomic DNA amplification (53.8%) (Figure 1), suggesting its important role in the development of CCA. TP gene located at 22q13.33 is an angiogenic factor, which promotes angiogenesis in vivo and stimulates the in vitro growth of a variety of endothelial cells. TP also has an enzymatic activity involved in pyrimidine metabolism. It catalyzes the phosphorolysis of thymidine to thymine and deoxyribose-1-phosphate and has a pro-angiogenic effect for which deoxyribose-1-phosphate may be responsible[24-26]. Since gene amplification is reported to play an important role in the initiation and progression of tumors[27], TP amplification and overexpression have been reported in many solid tumors with invasion and metastasis including CCA[28-30]. These observations indicate that the incidence of amplification and overexpression of TP is high in cancer and associated with carcinogenesis. Although we could not find any correlation between clinicopathological parameters and TP imbalance or impact of TP gene copy number on patient survival when treated with 5-fluorouracil (5-FU)-based chemotherapy (data not shown), its high amplification may be worth further investigation into its involvement in chemotherapeutic activity because normal TP protein activity is required for the activation of the cytotoxic activities of anti-tumor drug 5-FU. 5-FU-based chemotherapy is given to patients with advanced cancer and as an adjuvant treatment. 5-FU is a fluorinated pyrimidine that is metabolized intracellularly to its active form, fluorodeoxyuridine monophosphate (FdUMP), by TP to inhibit DNA synthesis. Our further studies will investigate whether TP expression also associates with chemosensitivity in CCA.
Microsatellite marker D22S423 was the most frequent locus loss (40%) among 9 loci, suggesting that this region may harbor a putative tumor suppressor gene (TSG), which plays a role in the development of CCA. LOH frequency at D22S423 was reported in 26.8% of informative cases in sporadic colorectal cancer[15]. Putative TSG located 1 cM distal to D22S423 are ST13 and EP300, however, the presence of unknown TSG located within this region cannot be excluded. ST13 encodes an adaptor protein that mediates the association of the heat shock proteins HSP70 and HSP90. The expression of ST13 is reported to be downregulated in colorectal carcinoma tissue compared with that in adjacent normal tissue, suggesting that ST13 is a candidate TSG[31]. Function and role of ST13 in tumor development is still unclarified. Further investigation into ST13 expression and its roles in carcinogenesis of CCA is needed.
D22S283 was deleted in 15 cases, which all had lym-phatic invasion, suggesting the deletion of unknown TSG, which functions on inhibiting invasion. D22S283 showed amplification in 27 out of 65 cases, indicating the existence of putative oncogene (s) at this location, for example, RAB member of RAS oncogene family, RNA binding motif protein 9, and eukaryotic translation initiation factor 3. On the other hand, some TSG at this location may amplify, leading to high expression which may result in good prognosis of patients with D22S283 amplification (Figure 3). Other putative TSG around D22S283 that play a role in apoptosis are caspase recruitment domain family, member 10 and phospholipase A2, group VI.
All 23 cases of BIK gene deletion were associated with lymphatic invasion. Many reports suggest that BCL2-interacting killer (apoptosis-inducing) functions as a proapoptotic protein, which enhances programmed cell death. Germain et al[32] identified BIK as an initiator of cytochrome C released from mitochondria operating from a location at the endoplasmic reticulum and activated caspases. Thus, role of BIK in pathogenesis and carcinogenesis of CCA needs further investigation. NF2 gene located at 22q12.2 produces the Merlin protein. It is thought to act as a tumor suppressor protein, however, the mechanism remains obscure. Xiao et al[33] have shown that merlin inhibits tumor cell proliferation and arrests cells at G1 phase, concomitant with decreased expression of cyclin D1, inhibition of CDK4 activity, and dephosphorylation of pRB. They suggested a unifying mechanism by which merlin inactivation might contribute to the overgrowth seen in both noninvasive and malignant tumors. DNA amplification of NF2 was found in 36.9 % of our CCA cases, hence, expression of NF2 may lead to significant reduction of proliferation and G0/G1 arrest in CCA cells.
The development of tumor is a multistep process that requires accumulated mutations or alterations of both oncogenes and tumor suppressor genes. It is generally believed that mutations in 5 to 10 genes are required for the development of a tumor. Thus, TP, BIK, NF2, and candidate genes on D22S423 and D22S283 are likely to play significant roles in carcinogenesis of liver fluke related CCA by this study. In addition, to our knowledge, we first propose that the D22S283 amplification acts as an independent indicator of favorable prognosis in liver fluke related CCA.
Footnotes
Supported by The Thailand Research Fund through The Royal Golden Jubilee PhD Program, Grant No. PHD/0037/2544 for Thanasai J and Limpaiboon T
S- Editor Pan BR L- Editor Zhu LH E- Editor Liu WF
References
- 1.Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994;305:241–252. doi: 10.1016/0027-5107(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 2.Ohshima H, Bandaletova TY, Brouet I, Bartsch H, Kirby G, Ogunbiyi F, Vatanasapt V, Pipitgool V. Increased nitrosamine and nitrate biosynthesis mediated by nitric oxide synthase induced in hamsters infected with liver fluke (Opisthorchis viverrini) Carcinogenesis. 1994;15:271–275. doi: 10.1093/carcin/15.2.271. [DOI] [PubMed] [Google Scholar]
- 3.Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32 Suppl:S82–S91. doi: 10.1093/jjco/hye134. [DOI] [PubMed] [Google Scholar]
- 4.Uttaravichien T, Bhudhisawasdi V, Pairojkul C, Pugkhem A. Intrahepatic cholangiocarcinoma in Thailand. J Hepatobiliary Pancreat Surg. 1999;6:128–135. doi: 10.1007/s005340050095. [DOI] [PubMed] [Google Scholar]
- 5.Horie S, Endo K, Kawasaki H, Terada T. Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma: relationship with p53 overexpression, Ki-67 labeling, and clinicopathological features. Virchows Arch. 2000;437:25–30. doi: 10.1007/s004280000201. [DOI] [PubMed] [Google Scholar]
- 6.Tannapfel A, Benicke M, Katalinic A, Uhlmann D, Köckerling F, Hauss J, Wittekind C. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–727. doi: 10.1136/gut.47.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limpaiboon T, Khaenam P, Chinnasri P, Soonklang M, Jearanaikoon P, Sripa B, Pairojkul C, Bhudhisawasdi V. Promoter hypermethylation is a major event of hMLH1 gene inactivation in liver fluke related cholangiocarcinoma. Cancer Lett. 2005;217:213–219. doi: 10.1016/j.canlet.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Limpaiboon T, Krissadarak K, Sripa B, Jearanaikoon P, Bhuhisawasdi V, Chau-in S, Romphruk A, Pairojkul C. Microsatellite alterations in liver fluke related cholangiocarcinoma are associated with poor prognosis. Cancer Lett. 2002;181:215–222. doi: 10.1016/s0304-3835(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 9.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Lee KT, Kim EK, Sohn TS, Heo JS, Choi SH, Choi DI, Lee JK, Paik SW, Rhee JC. Expression of cyclooxygenase-2 in cholangiocarcinoma: correlation with clinicopathological features and prognosis. J Gastroenterol Hepatol. 2004;19:582–588. doi: 10.1111/j.1440-1746.2003.03299.x. [DOI] [PubMed] [Google Scholar]
- 11.Koo SH, Ihm CH, Kwon KC, Lee JS, Park JW, Kim JW. Microsatellite alterations in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Genet Cytogenet. 2003;146:139–144. doi: 10.1016/s0165-4608(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 12.Momoi H, Okabe H, Kamikawa T, Satoh S, Ikai I, Yamamoto M, Nakagawara A, Shimahara Y, Yamaoka Y, Fukumoto M. Comprehensive allelotyping of human intrahepatic cholangiocarcinoma. Clin Cancer Res. 2001;7:2648–2655. [PubMed] [Google Scholar]
- 13.Shiraishi K, Kusano N, Okita S, Oga A, Okita K, Sasaki K. Genetic aberrations detected by comparative genomic hybridization in biliary tract cancers. Oncology. 1999;57:42–49. doi: 10.1159/000011999. [DOI] [PubMed] [Google Scholar]
- 14.Castells A, Gusella JF, Ramesh V, Rustgi AK. A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res. 2000;60:2836–2839. [PubMed] [Google Scholar]
- 15.Zhou CZ, Peng ZH, Zhang F, Qiu GQ, He L. Loss of heterozygosity on long arm of chromosome 22 in sporadic colorectal carcinoma. World J Gastroenterol. 2002;8:668–673. doi: 10.3748/wjg.v8.i4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis PP, Rogatto SR, Kowalski LP, Nishimoto IN, Montovani JC, Corpus G, Squire JA, Kamel-Reid S. Quantitative real-time PCR identifies a critical region of deletion on 22q13 related to prognosis in oral cancer. Oncogene. 2002;21:6480–6487. doi: 10.1038/sj.onc.1205864. [DOI] [PubMed] [Google Scholar]
- 17.Hirano A, Emi M, Tsuneizumi M, Utada Y, Yoshimoto M, Kasumi F, Akiyama F, Sakamoto G, Haga S, Kajiwara T, et al. Allelic losses of loci at 3p25.1, 8p22, 13q12, 17p13.3, and 22q13 correlate with postoperative recurrence in breast cancer. Clin Cancer Res. 2001;7:876–882. [PubMed] [Google Scholar]
- 18.Koo SH, Jeong TE, Kang J, Kwon KC, Park JW, Noh SM. Prognostic implications for gastric carcinoma based on loss of heterozygosity genotypes correlation with clinicopathologic variables. Cancer Genet Cytogenet. 2004;153:26–31. doi: 10.1016/j.cancergencyto.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Englefield P, Foulkes WD, Campbell IG. Loss of heterozygosity on chromosome 22 in ovarian carcinoma is distal to and is not accompanied by mutations in NF2 at 22q12. Br J Cancer. 1994;70:905–907. doi: 10.1038/bjc.1994.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Egami K, Sasajima K, Ghazizadeh M, Shimizu H, Watanabe H, Hasegawa H, Iida S, Matsuda T, Okihama Y, et al. Comparative study between DNA copy number aberrations determined by quantitative microsatellite analysis and clinical outcome in patients with stomach cancer. Clin Cancer Res. 2004;10:3013–3019. doi: 10.1158/1078-0432.ccr-03-0250. [DOI] [PubMed] [Google Scholar]
- 22.De Preter K, Speleman F, Combaret V, Lunec J, Laureys G, Eussen BH, Francotte N, Board J, Pearson AD, De Paepe A, et al. Quantification of MYCN, DDX1, and NAG gene copy number in neuroblastoma using a real-time quantitative PCR assay. Mod Pathol. 2002;15:159–166. doi: 10.1038/modpathol.3880508. [DOI] [PubMed] [Google Scholar]
- 23.Nigro JM, Takahashi MA, Ginzinger DG, Law M, Passe S, Jenkins RB, Aldape K. Detection of 1p and 19q loss in oligodendroglioma by quantitative microsatellite analysis, a real-time quantitative polymerase chain reaction assay. Am J Pathol. 2001;158:1253–1262. doi: 10.1016/S0002-9440(10)64076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima Y, Gotanda T, Uchimiya H, Furukawa T, Haraguchi M, Ikeda R, Sumizawa T, Yoshida H, Akiyama S. Inhibition of metastasis of tumor cells overexpressing thymidine phosphorylase by 2-deoxy-L-ribose. Cancer Res. 2004;64:1794–1801. doi: 10.1158/0008-5472.can-03-2597. [DOI] [PubMed] [Google Scholar]
- 25.Uchimiya H, Furukawa T, Okamoto M, Nakajima Y, Matsushita S, Ikeda R, Gotanda T, Haraguchi M, Sumizawa T, Ono M, et al. Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose. Cancer Res. 2002;62:2834–2839. [PubMed] [Google Scholar]
- 26.Akiyama S, Furukawa T, Sumizawa T, Takebayashi Y, Nakajima Y, Shimaoka S, Haraguchi M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004;95:851–857. doi: 10.1111/j.1349-7006.2004.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boon K, Eberhart CG, Riggins GJ. Genomic amplification of orthodenticle homologue 2 in medulloblastomas. Cancer Res. 2005;65:703–707. [PubMed] [Google Scholar]
- 28.Hotta T, Taniguchi K, Kobayashi Y, Johata K, Sahara M, Naka T, Watanabe T, Ochiai M, Tanimura H, Tsubota YT. Increased expression of thymidine phosphorylase in tumor tissue in proportion to TP-expression in primary normal tissue. Oncol Rep. 2004;12:539–541. [PubMed] [Google Scholar]
- 29.Sato J, Sata M, Nakamura H, Inoue S, Wada T, Takabatake N, Otake K, Tomoike H, Kubota I. Role of thymidine phosphorylase on invasiveness and metastasis in lung adenocarcinoma. Int J Cancer. 2003;106:863–870. doi: 10.1002/ijc.11315. [DOI] [PubMed] [Google Scholar]
- 30.Aishima S, Taguchi K, Sugimachi K, Asayama Y, Nishi H, Shimada M, Sugimachi K, Tsuneyoshi M. The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. Int J Surg Pathol. 2002;10:47–56. doi: 10.1177/106689690201000108. [DOI] [PubMed] [Google Scholar]
- 31.Wang LB, Zheng S, Zhang SZ, Peng JP, Ye F, Fang SC, Wu JM. Expression of ST13 in colorectal cancer and adjacent normal tissues. World J Gastroenterol. 2005;11:336–339. doi: 10.3748/wjg.v11.i3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Germain M, Mathai JP, Shore GC. BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J Biol Chem. 2002;277:18053–18060. doi: 10.1074/jbc.M201235200. [DOI] [PubMed] [Google Scholar]
- 33.Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, Jhanwar S, Testa JR. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


