Abstract
Congenital Disorder of Glycosylation type Ig (ALG12-CDG) is part of a group of autosomal recessive conditions caused by deficiency of proteins involved in the assembly of dolichol-oligosaccharides used for protein N-glycosylation. In ALG12-CDG, the enzyme affected is encoded by the ALG12 gene. Affected individuals present clinically with neurodevelopmental delay, growth retardation, immune deficiency, male genital hypoplasia, and cardiomyopathy. A total of six individuals have been reported in the literature. Here, we present an infant with rhizomelic short stature, talipes equinovarus, platyspondyly, and joint dislocations. The infant had marked under-ossification of the pubic bones. Exome sequencing was performed and two deletions, each resulting in a frameshift, were found in ALG12. A review of the literature revealed two infants with ALG12-CDG and a severe skeletal dysplasia, including under-ossification of cervical vertebrae, pubic bones, and knees; in addition to talipes equinovarus and rhizomelic short stature. The phenotype of the individual we describe resembles pseudodiastrophic dysplasia and we discuss similarities and differences between ALG12-CDG and pseudodiastrophic dysplasia. The differential diagnosis in selected undiagnosed skeletal dysplasias should include CDGs.
Abbreviations: CDG, Congenital Disorder of Glycosylation
Keywords: Congenital Disorder of Glycosylation, ALG12, CDG-Ig, ALG12-CDG, Severe skeletal dysplasia, Whole exome sequencing
1. Introduction
Congenital Disorders of Glycosylation (CDGs) encompass a diverse group of autosomal recessive metabolic disorders characterized by multi-systemic effects, including effects on the central and peripheral nervous systems [1]. CDG type I involves a defect in either the synthesis or the transfer of dolichylpyrophosphate-linked precursor oligosaccharide to target proteins and presents with under-glycosylated serum glycoproteins of hepatic origin. CDG type II affects N-linked sugar molecule chains [2]. Some of the CDGs have skeletal manifestations, and these were reviewed by Coman and colleagues in 2008 [3].
In this report, we present an individual with several skeletal abnormalities, some of which were suggestive of pseudodiastrophic dysplasia, in whom exome sequencing revealed two frameshift deletions in ALG12, the gene mutated in ALG12-CDG. We detail here the clinical and molecular findings in this individual and discuss the similarities and differences with pseudodiastrophic dysplasia.
2. Case report
The affected individual (International Skeletal Dysplasia Registry reference number R82-101) was a Hispanic female born at term to a 33 year-old G3P2Ab1 mother. The pregnancy was complicated by polyhydramnios, revealed by ultrasound at 30 weeks of gestation. At birth, her Apgar scores were 1/1/1 at 1, 5 and 10 min, and meconium was recovered from below the vocal cords. She required mechanical ventilation and subsequently died in the neonatal period.
Physical examination revealed a 3700 gram newborn with a low posterior hairline with redundant skin on the back of a short neck. She had normally positioned ears, midface hypoplasia, and a beaked nose with flared alae nasi. She had a broad, square trunk, a narrow pelvis, and apparent scoliosis. The lower extremities showed mild bowing bilaterally, and there was severe bilateral talipes equinovarus. The wrists exhibited ulnar deviation, redundant skin folds were noted at the wrists, the thumbs were proximally placed, and the second phalanges overlapped the third phalanges.
3. Radiology
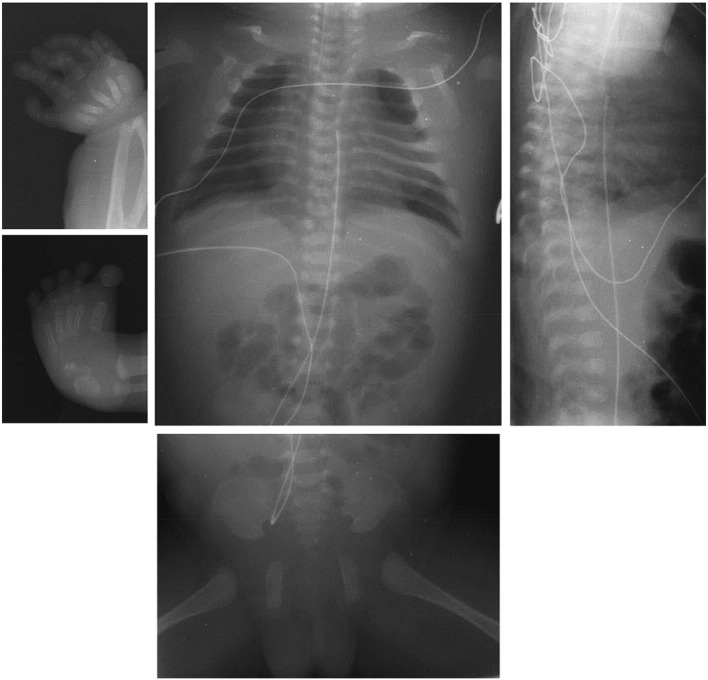
Radiographic examination (see Fig. 1) revealed 12 pairs of ribs, a single hemi-vertebra at T10, and numerous butterfly vertebrae. The metacarpals were small, and the hands had bilateral interphalangeal dislocations, making them reminiscent of pseudodiastrophic dysplasia. The pelvis exhibited slightly flat acetabular roofs, absent pubic bone ossification, and narrow sacrosciatic notches. Radial bowing was noted. There was bilateral duplication of the talus with punctate calcification of one center. The tibiae, fibulae, and femora were normal, although the distal femoral epiphyses were well-ossified for a newborn.
Fig. 1.
Radiographs of the affected individual. Radiographs show the butterfly vertebrae and the T10 hemivertebra. We also see small metacarpals and bilateral interphalangeal dislocations and radial bowing. The pelvis radiograph shows flat acetabular roofs, absent pubic bone ossification and narrow sacrosciatic notches.
4. Materials and methods
4.1. Histology
Bone histology was performed according to standard methods.
4.2. Genetic analyses
Exome sequencing was performed on a research basis as described previously [4]. Essentially, DNA was fragmented and exon-coding regions were selected using an in-house-developed capture reagent from Roche NimbleGen (Roche NimbleGen, Madison, WI) and sequenced on the Illumina HiSeq 2000 (Illumina, San Diego, CA). Read alignment and variant calling were performed using a pipeline of freely available software [4], and autosomal recessive coding variants that were rare (minor allele frequency < 1%) or novel were preferentially explored. For Sanger sequencing, amplicons were generated using 0.5 ng/μl of genomic DNA and TaqMan polymerase (ABI, Life Technologies, Carlsbad, CA) using the manufacturer's protocol. Primers used for detecting the ALG12 variants were for exons 2 and 3; Fwd: 5′-GTGGAGTGGCAGTGCTAACG-3′, Rev: 5′-ACCCGATGACACCACAGTC-3′; and for exons 8 and 9: Fwd:5′-TGAACAGCAGGTTATGCTCC3′, Rev: 5′-ATGAGAGCTGGTGGTCCTG-3′. Products were sequenced at Beckman Coulter Genomics (Danvers, MA).
5. Results
5.1. Histological analysis
Histological examination of the bones revealed a normal reserve zone and normal appearing bone (data not shown). Within the growth plates, there was an increased distance between the proliferative and hypertrophic zones, and some hypertrophic cells extended into the primary trabeculae. The histological phenotype was not diagnostic of any specific skeletal dysplasia.
5.2. Exome and Sanger sequencing
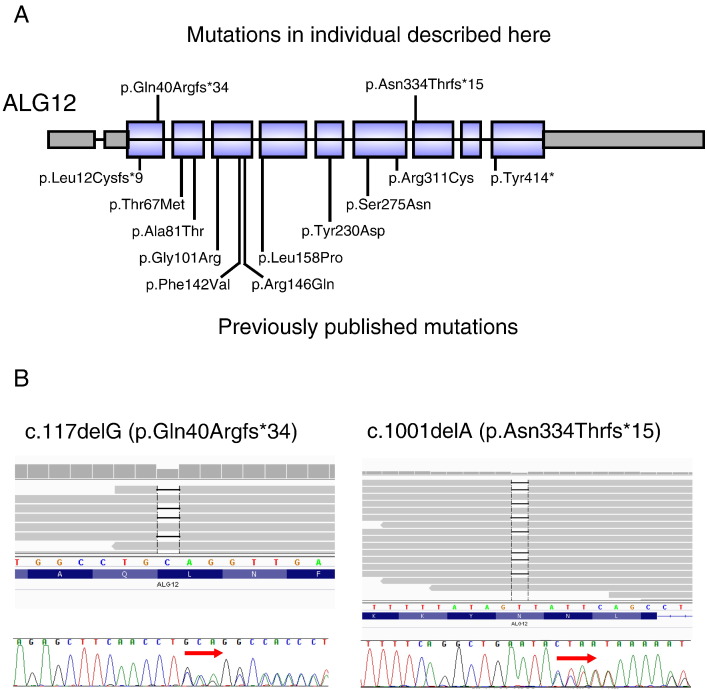
Exome sequencing revealed rare variants in 175 known disease-causing genes. After removal of false positive calls and correlation with known phenotypes, the ALG12 variants were considered the best candidates because they both resulted in frameshifts and because the skeletal phenotype seen in this patient corresponded well with ALG12-CDG. The ALG12 (RefSeq# NM_024105.3) mutations were c.1001delA (p.Asn334Thrfs*15) and c.117delG (p.Gln40Argfs*34). These mutations were confirmed by Sanger sequencing (Fig. 2). Cells for sialotransferrin studies to biochemically confirm the genetic data are not available.
Fig. 2.
ALG12 with exons, introns, and location of mutations in the affected individual. A) Location of the mutations we identified. The diagram also illustrates the exonic structure of ALG12, with the introns not drawn to scale. B) Mutations identified, shown on the exome alignment, and on Sanger sequencing chromatograms.
6. Discussion
ALG12-CDG was first described by Chantret et al. in 2002, presenting in a Tunisian infant with consanguineous parents [1]. She presented with hypotonia; dysmorphic facial features including large ears, thin upper lip, and prominent forehead; failure to thrive; severe psychomotor retardation; and progressive microcephaly. She also experienced recurrent infections with hypogammaglobulinemia. Skin fibroblasts had a reduced capacity to add the eighth mannose residue onto the dolichol-PP-oligosaccharide precursor required for protein glycosylation, and a homozygous point mutation was found in the human homolog of yeast gene ALG12, which encodes dolichyl-P-Man:Man7GlcNAc2-PP-dolichyl α6-mannosyltransferase.
Early on, there were no reported cases of ALG12-CDG in which the affected individual was noted to have skeletal abnormalities. However, in 2007, Kranz et al. reported on a brother–sister pair with compound heterozygosity for mutations in ALG12 who displayed features of a unique skeletal dysplasia in addition to cardiomyopathy, male genital malformations, and early death [5]. This sibling pair displayed many features previously found in ALG12-CDG, including hypogammaglobulinemia, hypotonia, failure to thrive, developmental delay, microcephaly, and a thin upper lip. In addition, they displayed numerous skeletal abnormalities, including talipes equinovarus, short flared ribs, ulnar deviation of the wrists, and diminished ossification of the pubic bones, knee epiphyses, tali, and temporal and occipital bones. The brother's humerus, radius, femur, and tibia were less than the 5th percentile for corrected gestational age, and the sister's forearms, lower legs, and humeri were short on 27-week ultrasound [5]. While the brother died at age 23 months due to sepsis secondary to hypogammaglobulinemia, the sister died at age 67 days secondary to cardiomyopathy. Whereas the child described by Chantret et al. had a normal brain MRI [1], the individuals described by Kranz et al. had abnormal neuroanatomy. Head MRIs in this sibling pair revealed numerous abnormalities, including pachygyria, hypoplastic cerebellar vermis, ventricular enlargement and prominence, delayed myelination, and a prominent cavum septum pellucidum [5].
Some of the findings in the individuals described by Kranz et al. and the individual we describe resemble those of pseudodiastrophic dysplasia. Pseudodiastrophic dysplasia is a skeletal dysplasia characterized by distinct radiographic, clinical, and histologic features. Individuals present with rhizomelic limb shortening and bilateral clubfoot deformity as well as proximal interphalangeal joint dislocations, elbow dislocations, platyspondyly, and scoliosis [6]. It was first described by Burgio et al. in 1974, when it occurred in two infant sisters [7]. Both sisters died of unexplained hyperthermia while still in infancy, one in the neonatal period. Since then, several other cases have been reported. Thus far, 11 individuals with pseudodiastrophic dysplasia have been described [6], [8], [9], [10], [11]. Due to its occurrence in sibling pairs and consanguinity in one family [11], pseudodiastrophic dysplasia is presumed to have autosomal recessive inheritance [6]. Some affected individuals have had abnormal urinary mucopolysaccharide testing; however, a causative gene has not been identified [6], [10], so mechanistic comparisons between pseudodiastrophic dysplasia and ALG12-CDG will be possible only after the molecular basis is known. Additional clinical features reported include abnormalities of the acetabulum [6], [11], neonatal contractures [6], and blue sclerae [6], [10]. Fischetto et al. described an individual with camptodactyly of the fifth finger, hypoplastic nails, congenital heart disease, and omphalocele [10]. The individuals described by Burgio et al. [7] and Fischetto et al. [10] suffered from episodes of hyperthermia surrounding their deaths. Both the individual described by Fischetto et al. [10] and one individual described by Eteson et al. [6] died of cardiopulmonary arrest.
Whereas the individuals described by Kranz et al. had generalized skeletal abnormalities that did not point to any particular dysplasia, the combination in the individual we describe, of interphalangeal dislocations, scoliosis, talipes equinovarus, rhizomelic limb shortening, midface hypoplasia, short metacarpals, and a somewhat horizontal acetabular roof suggested a possible clinical diagnosis of pseudodiastrophic dysplasia [6]. The patient also displayed ulnar deviation of the wrists, a finding seen in ALG12-CDG but not typical in pseudodiastrophic dysplasia [5]. A summary comparing the skeletal findings observed in individuals with a molecular diagnosis of ALG12-CDG, a clinical diagnosis of pseudodiastrophic dysplasia, and the individual described here is displayed in Table 1. Both the individuals described by Chantret et al. and Zdebska et al., as well as the individuals described by Kranz et al., exhibited both motor and developmental delay. In contrast, while two of the pseudodiastrophic dysplasia individuals described by Eteson et al. exhibited gross motor delay, both had normal language development [6].
Table 1.
Radiological findings in pseudodiastrophic dysplasia, the individual described here, and previously reported individuals with CDG-Ig.
| Trait | Diastrophic dysplasia | Pseudodiastrophic dysplasia | Individual described here | Other individuals with CDG-Ig |
|---|---|---|---|---|
| Rhizomelic limb shortening | + | + | + | + |
| Dislocated hips and elbows | + | + | − | − |
| Interphalangeal dislocations | − | + | + | − |
| Scoliosis | + | + | + | − |
| Talipes equinovarus | + | + | + | + |
| Short, flared ribs | + | + | − | + |
| Platyspondyly | − | + | − | − |
| Short metacarpals | + | + | − | + |
| Horizontal acetabular roof | − | + | + | − |
| Ulnar deviation of wrists | − (Ulnar deviation of fingers) | − | + | + |
| Delayed ossification | − (Early calcification of carpal bones) | − | − | + |
| Talus duplication | − | − | + | − |
| Ears | Cystic ear swelling | Large malformed earlobes with folded superior helix | − | − |
| Hypertelorism | − | + | − | NA |
| Midface hypoplasia | − | + | + | NA |
| Anteverted nostrils | − | + | + | NA |
| Cleft palate | + | + | − | − |
| Unexplained fever | − | + | − | − |
In pseudodiastrophic dysplasia bone histology, there are shortened, irregular proliferative columns, some of which are interrupted by fibrovascular cysts, which extend beyond the resting cartilage. The primary trabeculae in pseudodiastrophic dysplasia are short, and exhibit a thickened cartilaginous center. In our patient, histology revealed increased distance between the proliferative and hypertrophic zones, and some hypertrophic cells extending into the primary trabeculae, which are not typical of either diastrophic or pseudodiastrophic dysplasia.
6.1. Conclusion
In conclusion, we describe an individual with ALG12-CDG who had some skeletal manifestations reminiscent of pseudodiastrophic dysplasia. In the context of exome sequencing that revealed mutations in ALG12, clinical and histological features distinguished the case from pseudodiastrophic dysplasia. Given the frequent skeletal abnormalities observed in CDGs, the differential diagnosis in some unsolved skeletal dysplasia cases should include CDGs [3].
Acknowledgments
The authors have no conflict of interest to declare. We thank Yuqing Chen for technical assistance, and Shalini N. Jhangiani for exome sequencing coordination. Funding was provided by NIH grants P01 HD22657 (BHL, DHC), P01 HD070394 (BHL), R01 AR062651 (DHC), R01 DE019567 (DHC), U54 HG006542 (RAG) and U54 HG003273-09 (RAG). Funding was also provided by The Rolanette and Berdon Lawrence Bone Disease Program of Texas (BL). PMC was supported by a CIHR clinician–scientist training award and JTL is supported by Ruth L. Kirschstein National Research Service Award F30 MH098571-01.
References
- 1.Chantret I., Dupre T., Delenda C., Bucher S., Dancourt J., Barnier A., Charollais A., Heron D., Bader-Meunier B., Danos O., Seta N., Durand G., Oriol R., Codogno P., Moore S.E. Congenital disorders of glycosylation type Ig is defined by a deficiency in dolichyl-P-mannose:Man7GlcNAc2-PP-dolichyl mannosyltransferase. J. Biol. Chem. 2002;277:25815–25822. doi: 10.1074/jbc.M203285200. [DOI] [PubMed] [Google Scholar]
- 2.Woods A.G., Woods C.W., Snow T.M. Congenital disorders of glycosylation. Adv. Neonatal Care. 2012;12:90–95. doi: 10.1097/ANC.0b013e318241cb20. [DOI] [PubMed] [Google Scholar]
- 3.Coman D., Irving M., Kannu P., Jaeken J., Savarirayan R. The skeletal manifestations of the congenital disorders of glycosylation. Clin. Genet. 2008;73:507–515. doi: 10.1111/j.1399-0004.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Laine C.M., Joeng K.S., Campeau P.M., Kiviranta R., Tarkkonen K., Grover M., Lu J.T., Pekkinen M., Wessman M., Heino T.J., Nieminen-Pihala V., Aronen M., Laine T., Kroger H., Cole W.G., Lehesjoki A.E., Nevarez L., Krakow D., Curry C.J., Cohn D.H., Gibbs R.A., Lee B.H., Makitie O. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kranz C., Basinger A.A., Gucsavas-Calikoglu M., Sun L., Powell C.M., Henderson F.W., Aylsworth A.S., Freeze H.H. Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am. J. Med. Genet. A. 2007;143A:1371–1378. doi: 10.1002/ajmg.a.31791. [DOI] [PubMed] [Google Scholar]
- 6.Eteson D.J., Beluffi G., Burgio G.R., Belloni C., Lachman R.S., Rimoin D.L. Pseudodiastrophic dysplasia: a distinct newborn skeletal dysplasia. J. Pediatr. 1986;109:635–641. doi: 10.1016/s0022-3476(86)80227-x. [DOI] [PubMed] [Google Scholar]
- 7.Burgio G.R., Belloni C., Beluffi G. Pseudodiastrophic dwarfism. Study of 2 newborn sisters. Arch. Fr. Pediatr. 1974;31:681–696. [PubMed] [Google Scholar]
- 8.Canki N., Sernec-Logar B., Prodan M., Pintar L. Pseudodiastrophic dwarfism: a case report. J. Genet. Hum. 1979;27:247–252. [PubMed] [Google Scholar]
- 9.Canki-Klain N., Stanescu V., Bebler P., Maroteaux P. Pseudodiastrophic dysplasia evolution with age and management. Report of two new cases and review of the literature. Ann. Genet. 1990;33:129–136. [PubMed] [Google Scholar]
- 10.Fischetto R., Causio F., Corso G., Lillo V., Natale B., Papadia F. Pseudodiastrophic dysplasia type Burgio in a newborn. Am. J. Med. Genet. 1997;71:222–225. doi: 10.1002/(sici)1096-8628(19970808)71:2<222::aid-ajmg20>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand J.G., Tyazi A., Zaoui C., Vandevelde M.F., Desmettre C., Ramaherisson P., Cuingnet P.H., Noel J.L., Bertrand J. Pseudo-diastrophic dysplasia. Ann. Pediatr. (Paris) 1991;38:19–22. [PubMed] [Google Scholar]