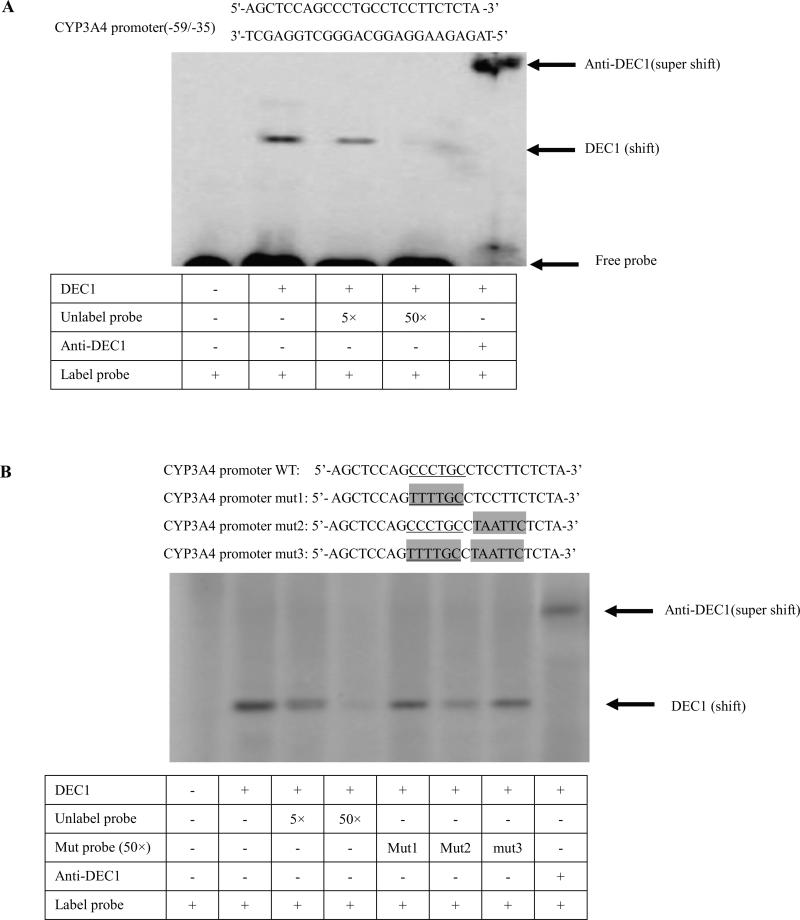
Fig. 5. DEC1 binding to the CCCTGC sequence in the proximal promoter of CYP3A4 by EMSA.
(A) DEC1 binding to region (−59/−35) of CYP3A4 proximal promoter. (B) DEC1 binding to the CCCTGC sequence of CYP3A4 proximal promoter. HepG2 cells were plated in six-well plates and transfected with Flag-DEC1 or Flag CMV2 800 ng/well overnight. Nuclear extracts were prepared with a nuclear extraction kit (Active Motif). Nuclear proteins (10 μg) were incubated with 32P-labelled oligonucleotides (5'-AGCTCCAGCCCTGCCTCCTTCTCTA -3’) in a final volume of 10 μl containing 1 × DNA-binding buffer. For competition experiment, nuclear extracts were incubated with excess unlabelled wild type probe (5×, 50×) or each unlabelled mutated probe (mut1, 2, 3) (50×) and then mixed with the radioactive-belled probe. For supershift assays, an anti-DEC1 antibody was added after the nuclear extracts were incubated with the radioactive-labelled probe. The protein-DNA complexes were resolved on 6% polyacrylamide gel and visualized by Typhoon 8600 Variable Mode Imager. Three independent experiments were performed.