Abstract
The deposition of aerosol in the human lung occurs mainly through a combination of inertial impaction, gravitational sedimentation, and diffusion. For 0.5- to 5-μm-diameter particles and resting breathing conditions, the primary mechanism of deposition in the intrathoracic airways is sedimentation, and therefore the fate of these particles is markedly affected by gravity. Studies of aerosol deposition in altered gravity have mostly been performed in humans during parabolic flights in both microgravity (μG) and hypergravity (∼1.6G), where both total deposition during continuous aerosol mouth breathing and regional deposition using aerosol bolus inhalations were performed with 0.5- to 3-μm particles. Although total deposition increased with increasing gravity level, only peripheral deposition as measured by aerosol bolus inhalations was strongly dependent on gravity, with central deposition (lung depth<200 mL) being similar between gravity levels. More recently, the spatial distribution of coarse particles (mass median aerodynamic diameter≈5 μm) deposited in the human lung was assessed using planar gamma scintigraphy. The absence of gravity caused a smaller portion of 5-μm particles to deposit in the lung periphery than in the central region, where deposition occurred mainly in the airways. Indeed, 5-μm-diameter particles deposit either by inertial impaction, a mechanism most efficient in the large and medium-sized airways, or by gravitational sedimentation, which is most efficient in the distal lung. On the contrary, for fine particles (∼1 μm), both aerosol bolus inhalations and studies in small animals suggest that particles deposit more peripherally in μG than in 1G, beyond the reach of the mucociliary clearance system.
Key words: : microgravity, aerosol bolus, gamma scintigraphy
Introduction
The deposition of aerosol in the human lung occurs mainly through a combination of inertial impaction, gravitational sedimentation, and Brownian diffusion. Inertial impaction causes most of the large particles (>5 μm) to deposit in the upper respiratory tract (which extends from the lips to the glottis) and the large conducting airways; sedimentation mainly affects particles in the size range 1–8 μm and results from the gravitational settling of the particles in the airspaces; whereas Brownian diffusion is most effective for small particles (<1 μm), which deposit mainly in the alveolar region of the lung. Of these three mechanisms, sedimentation is the only gravity-dependent process and is thus expected to be altered in reduced gravity.
Although there is little, if any, structural difference between the top and the bottom of the healthy human lung, there are large regional differences in ventilation caused by the effect of gravity.(1) Indeed, because the lung distorts under its own weight, the alveoli at the base of the lung are relatively compressed compared with apical alveoli, and because poorly expanded alveoli are more compliant, ventilation is greatest near the bottom of the lung and becomes progressively lower near the apex. Changes in the gravity level affect the distortion of the lung under its own weight and, as a consequence, affect the distribution of ventilation. These changes are also expected to affect the distribution and deposition of inhaled particles.
The first investigations on the effect of gravity on aerosol deposition were theoretical analyses(2,3) that focused on the change in deposition of aerosols inhaled on the surface of the moon. These analyses predicted a lower overall deposition in reduced gravity, but an increase in alveolar deposition. Few experimental studies have been performed on the effect of gravity on aerosol inhalation, most likely because of the challenging environment in which this type of study needs to be performed. The first experimental study in altered gravity was performed by Hoffman and Billingham(4) in the mid-1970s. It was not until the mid-1990s that more extensive measurements of aerosol deposition in reduced gravity were made.(5–14) To date, all experimental studies on aerosol inhalation in reduced gravity have been performed during short periods of reduced gravity achieved during parabolic flights. The aim of this article is to review these experimental studies and highlight the role of gravity in the transport and deposition of inhaled particles in the human lung.
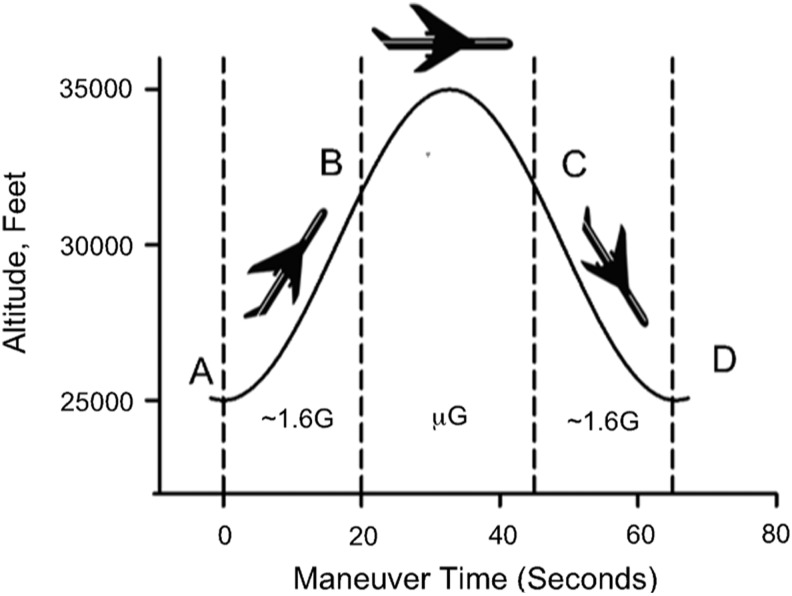
Reduced Gravity During Parabolic Flights
Studies on the effect of gravity on aerosol deposition in the human lung have been performed during parabolic flights aboard the National Aeronautics and Space Administration (NASA) Microgravity Research Aircraft. During these flights, the aircraft typically climbs to an altitude of ∼25,000 ft with the cabin pressurized to ∼600 Torr. A “roller coaster” flight profile is then performed (Fig. 1). The aircraft is pitched up at a 45° nose-high attitude to generate ∼1.6G (hypergravity; point A in Fig. 1). Then the nose is lowered to abolish wing lift, and thrust is reduced to produce a reduced gravity level (point B in Fig. 1). The maneuver can be modified to produce any level of gravity level less than normal gravity (1G). The most common levels achieved are microgravity (μG), ∼1/6G representative of lunar gravity, or ∼1/3G representative of Martian gravity. The specific flight profile is then maintained until the aircraft nose is 45° below the horizon (point C in Fig. 1). In this manner, microgravity, lunar gravity, or Martian gravity is upheld for ∼25, ∼30, or ∼40 sec, respectively. This is then followed by a pullout averaging ∼1.6G causing the nose to pitch up to a 45° nose-high attitude and allowing the cycle to be repeated (point D in Fig. 1). In a typical flight, these parabolic profiles are repeated ∼40 times.
FIG. 1.
Typical microgravity trajectory flown during parabolic flight. See text for details.
Overall Deposition in Reduced Gravity
In the first ever experimental study of aerosol deposition in altered gravity, Hoffman and Billingham studied the deposition of 2-μm-diameter particles in three subjects for gravity (G) level ranging from 0 to 2G. A fixed volume (5 L) of aerosol was stored in a spirometer before being rebreathed by the subjects in five breaths lasting 20 sec (i.e., at a flow rate of 0.5 L/sec and tidal volume of 1 L). Deposition was determined as the difference between particle counts made at the beginning and the end of the series of breaths by pumping ∼500 mL of aerosol through a NASA-developed aerosol counter. Data showed an almost linear increase in deposition with increasing G level (Fig. 2A, closed triangles). These data strongly suggested that gravitational sedimentation is the most efficient deposition mechanism for this particle size. Indeed, deposition by gravitational sedimentation is proportional to the terminal settling velocity of the particles, which in turn is directly proportional to gravitational acceleration.(15) Therefore, deposition by gravitational sedimentation is directly proportional to the gravity level.
FIG. 2.
Total deposition of aerosol particles (means±SD) as a function of G level. (A) Experimental data from Darquenne et al.(6) (open symbols, N=4) and Hoffman and Billingham(4) (closed symbols, N=3). Modified from Darquenne et al.(6) (B) Differences between experimental data and numerical predictions from 1D models. *Significantly different from experimental data (p<0.05). There were no significant differences between experimental values and numerical predictions in 1G and 1.6G. Significant differences were found in μG for 0.5-, 1-, and 2-μm particles.
Darquenne et al.(6) extended these studies to different particle sizes ranging between 0.5 and 3 μm, making measurements in μG, 1G, and ∼1.6G (Fig. 2A, open symbols). Subjects were breathing aerosols at a constant flow rate (0.4 L/sec) and tidal volume (0.75 L) while aerosol concentration and flow rate were measured at the mouth with an in-line photometer and pneumotachograph, respectively. Similar to Hoffman and Billingham's study,(4) they found a linear increase in deposition with increasing G level for both 2- and 3-μm particles. However, for the smaller particles they studied (0.5 and 1 μm), the increase in deposition was less between μG and 1G than between 1G and 1.6G. Assuming that a linear relationship between 1G and 1.6G defined the gravitational influence, the extrapolation to μG would predict deposition that is much less than that measured. Comparison between experimental measures and predictions from one-dimensional (1D) models of aerosol deposition in the intrathoracic airways at each gravity level(6,16) confirmed this trend where predictions correlated well with experimental results at 1G and 1.6G, but underestimated deposition in μG (Fig. 2B). The effect was greatest for the small particles for which deposition by impaction is negligible. Because there is no sedimentation in μG, only diffusion is apparently left to account for the measured deposition. However, deposition by Brownian diffusion on its own cannot explain the high deposition measured in μG.(6) The authors speculated that some form of “enhanced diffusion” was occurring that increased the deposition of the smallest particles studied, probably in the alveolar region of the lung. This was likely a result of the previously unaccounted for nonreversibility of flow in the alveolar regions of the lung, which leads to a net transport of particles in the direction of the alveoli during tidal breathing.(17,18) The absence of the removal of particles by sedimentation resulted in higher airway concentrations of particles than would otherwise be the case, enhancing aerosol transport to the alveolar region of the lung. The same effect was observed in a subsequent study(9) where deposition was measured in lunar gravity (∼1/6G). Furthermore, deposition in 1/6G was not significantly different from deposition measured in μG. This suggests that studies in μG likely provide a good surrogate for investigating potential effects of inhaled particulates to the health of human explorers to the moon. Finally, the lack of effect shown in Figure 2B for 3-μm particles suggests that the model accurately predicts deposition at all G levels for this particle size. According to the model, impaction accounts for ∼75% of predicted deposition in μG, but only for ∼21% in normal gravity where deposition by sedimentation accounts for ∼77% of total deposition (data not shown), suggesting a strong gravitational effect even for 3-μm particles.
Regional Deposition in Reduced Gravity
Aerosol bolus inhalations were then performed in an attempt to better understand the total deposition measurements by probing deposition at different depths within the lung. The aerosol bolus test consists in inserting a small amount of aerosol, a bolus, surrounded by clean filtered air at a predetermined point in the subject's inspiratory volume and analyzing the distribution of the aerosol bolus during the subsequent expiration. A bolus inserted early in the inspiratory volume probes the lung periphery, whereas a bolus inserted late in the inspiratory volume probes more proximal lung regions. The depth reached by the bolus is usually referred to as the penetration volume (Vp) and is defined as the volume of air inhaled from the mode of the bolus (i.e., the volume at which the maximum aerosol concentration occurs) to the end of inspiration.(19) Measurements were made in μG, 1G, and ∼1.6G with 0.5-, 1-, and 2-μm-diameter particles for penetration volumes ranging between 150 and 1,500 mL.(10,11) Similar to previous studies in 1G,(20–22) aerosol deposition increased linearly with depth of inhalation of the bolus within the lung (i.e., Vp) at all gravity levels studied (Fig. 3). Whereas particle size affected deposition for Vp>200 mL for data collected in 1G and 1.6G (Fig. 3B and C), there was no difference in deposition between these particle sizes at all penetration volumes when data were collected in μG (Fig. 3A). These observations are consistent with the previous study of total aerosol deposition,(6) which showed no size dependence in deposition over this range of particle sizes in μG. As for the total deposition study, the aerosol bolus data strongly suggest that differences observed between particle sizes in 1G and 1.6G result from the difference in settling velocities, i.e., from differences in the rate of deposition by gravitational sedimentation. It should also be noted that, for a given particle size, deposition was not significantly different from one G level to the other at shallow penetration volume (i.e., Vp=200 mL), confirming that deposition by sedimentation is a process that takes place primarily in the periphery of the lung.
FIG. 3.
Aerosol bolus deposition (means±SD, N=4) of 0.5-, 1-, and 2-μm-diameter particles as a function of penetration volume (Vp). (A) μG; (B) 1G; (C) 1.6G. *Significantly different from 1 μm (p<0.05). From Darquenne et al.(11)
The increase in deposition with increasing penetration volume in the absence of gravity (Fig. 3A) suggests that mechanisms other than gravitational sedimentation also significantly affect deposition in the human lung. As mentioned previously, predictions based on 1D models of aerosol transport that incorporate the three main mechanisms of deposition (i.e., impaction, sedimentation, and diffusion) could not account for deposition measured in μG.(6) Other potential contributors to aerosol deposition may include mixing induced by the nonreversibility of the flow in the airways and/or by cardiogenic mixing resulting from the physical motion of the heart that generates an oscillating motion of air in the lungs.(23–25) The effect of cardiogenic mixing on aerosol bolus behavior was previously investigated by Scheuch and Stahlhofen,(26) who performed bolus inhalations of 1-μm-diameter particles on a subject at rest and after exercise when the heart rate was increased by more than a factor of 2. They showed that the motion of the heart increased aerosol deposition and that this increase was more obvious at shallow penetration volumes. To further assess the potential effect of cardiogenic mixing, aerosol bolus tests that included a breath hold after the inhalation of the bolus were performed both in μG and in 1G.(5,13) Data were obtained with 1-μm particles at a penetration volume of 150 and 500 mL and breath holds of 0, 3, and 5 sec(5) and with 0.5-μm particles at a penetration volume of 300 and 1,200 mL and breath holds of 0, 5, and 10 sec(13) (Fig. 4). There was a strong effect of gravity with increasing breath-hold time on aerosol deposition in 1G for all cases studied. In μG, deposition was smaller than in 1G, but still showed a significant increase in the deposition of 0.5-μm particles with increasing breath-hold time for experiments performed with up to 10-sec breath holds (Fig. 4C and D). Such an increase is compatible with an effect of cardiogenic mixing on deposition as suggested by Scheuch and Stahlhofen.(26) Indeed, as there was no inspiratory or expiratory flow in the airways during the breath holds, neither impaction nor flow irreversibility could have caused the observed increase in deposition. Sedimentation was not a factor either because of the absence of gravity. It should be noted that whereas heart rate is not affected by μG, stroke volume is significantly higher in μG compared with 1G(27); therefore, one would expect cardiogenic mixing to be larger in μG than in 1G. Further, in the lung periphery where airspaces are small, Brownian diffusion may be non-negligible. Indeed, the Brownian displacements of 0.5-μm particles average ∼20 μm in 1 sec.(11) Therefore, during a 10-sec breath hold, particles can diffuse over a distance that is comparable to the diameter of an alveolus and may have contributed to the increased deposition measured in the lung periphery (Fig. 4D). Although these experiments suggest a potential significant effect of cardiogenic mixing on aerosol deposition, this effect is small when compared with that of gravity. Finally, it should also be kept in mind that, even in a reduced gravity environment, cardiogenic mixing would only be significant when other mechanisms of deposition such as Brownian diffusion and inertial impaction are small, i.e., for particles around 0.5–1 μm.
FIG. 4.
Aerosol bolus deposition as a function of breath-hold time. Data are means±SD, averaged over four (A, B) and eight subjects (C, D), respectively. Data are shown for μG (open circles) and 1G (closed circles) conditions. For clarity purposes, only one-sided error bars are shown on the figure. *Significantly different from μG. +Significant differences between breath holds in both μG and 1G (p<0.05). Modified from Darquenne et al.(5) and Prisk et al.,(13) with permission from Elsevier for the latter.
Implications for Space Exploration
Although there is no doubt that the inhalation and deposition of small particles in the lungs are a health concern here on Earth, long-term spaceflight represents a situation in which aerosol deposition may also be an important health consideration. In a spacecraft environment such as the International Space Station (ISS) or a future lunar or Martian habitat, the potential for significant airborne particle loads is high, as the environment is closed and sedimentation is either absent (as on the ISS) or largely reduced (as in the case of a lunar or Martian habitat). In particular, dust on the surface of the moon is thought to be toxic due to the fracturing processes involved in its formation, the chemical composition of the dust, and the radiation environment of the lunar surface.(28) Reports from the Apollo missions describe lunar dust as pervasive. Once tracked inside the lunar module, some of the dust easily became airborne, irritating lungs and eyes.(29) The size analysis of lunar soil samples collected during the Apollo missions showed that the samples contained a significant amount of respirable dust.(30) Indeed, the percent weight of the <1-cm fraction of bulk regolith (i.e., loose, heterogeneous material covering rock) for which particle size was <1 μm ranged from 4% to 25%.
The current proposed design of the lunar habitat calls for a reduced pressure environment (∼390 mmHg, 32% O2) resulting in a gas density of ∼53% that of sea-level air.(31,32) Studies in 1G of aerosol transport while breathing low-density gas [80% helium, 20% oxygen (80:20 heliox); about one third of sea-level air density] showed a reduction in deposition in the upper respiratory tract and large airways, and an increase in deposition in the peripheral lung.(33–35) The combination of reduced gravity and reduced gas density on aerosol deposition that was representative of a lunar habitat was investigated with the hypothesis that such combination would increase the deposition of aerosol particles in the peripheral lung in a synergistic manner. Similar to previous experiments performed with air,(9,11) deposition of 1-μm-diameter particles while breathing the low-density gas was less in μG than in 1G (Fig. 5), consistent with the absence of removal by sedimentation in μG. Although deposition in the low-density gas was less than that measured in air both in the central airways (Vp=200 mL) and in the proximal acinar region (Vp=500 mL), the reduction did not reach significance. This contrasts with a previous study of aerosol transport in 1G that showed a reduction in deposition in the upper respiratory tract and large airways while breathing heliox (80:20) instead of air, and a slight increase in deposition in the peripheral lung.(34) Reduction in deposition in the upper respiratory tract and large airways was attributed to the reduction in deposition by turbulent mixing as heliox reduces turbulent flow in the trachea and secondary transitional flows in the conducting airways because of its reduced density. Data shown in Figure 5 suggest that the reduction in gas density for the proposed lunar habitat is insufficient to significantly reduce turbulence in the upper respiratory tract and large conducting airways, and hence for deposition to be significantly reduced compared with sea-level conditions.(14) Therefore, gravity, and not gas properties, is the dominant factor in deposition in the lung.
FIG. 5.
Effect of reduced density and reduced gravity on aerosol deposition of 1-μm-diameter particles in four subjects (means±SD). For clarity purposes, only one-sided error bars are shown on the figure. Arrow shows the increase in penetration volume required to obtain a similar deposition level in 1G and under lunar habitat conditions. *#Significantly different compared with air and low-density deposition in 1G, respectively (p<0.05). Modified from Darquenne and Prisk.(14)
Although overall deposition is less in reduced gravity than in 1G, a particular deposition level is reached at a greater penetration volume in reduced gravity than in normal gravity, suggesting that relative deposition in the lung periphery is increased in reduced gravity. For example, for 1-μm-diameter particles, a level of 20% deposition was reached by Vp=240 mL in normal gravity, but not until Vp=575 mL under lunar habitat conditions (Fig. 5). Thus, particles that would normally be deposited in the central airways in normal gravity remained in suspension in the lunar environment. Particles were then available to be transported deeper into the lung. These observations may have important consequences on clearance rates of deposited particles. Indeed, a significant fraction of particles that would have deposited in the conducting airways in normal gravity and cleared within 1 day by mucociliary transport will deposit in the nonciliated regions of the lungs in lunar gravity, where clearance proceeds by much slower processes.(36) Therefore, particle retention—the difference between the number of deposited particles and the number of cleared particles—will likely be higher in reduced gravity.
Retention of Deposited Particles in Reduced Gravity
Besides the nature of the particles themselves, the toxicity of inhaled particles depends not only on the sites of deposition in the lung, but also on how fast deposited particles can be removed or translocated. Particles that deposit in the conducting airways are mainly removed by mucociliary clearance, whereas most of the particles that deposit in the alveolar region are phagocytized and cleared by alveolar macrophages. The rate of these clearance mechanisms differs by several orders of magnitude, with mucociliary clearance being very much the faster process (half-life of hours/day versus months/years).(37,38) Although the effect of μG on mucociliary clearance and alveolar macrophage phagocytosis is unknown,(39) it is likely that, even in μG, mucociliary clearance is a faster mechanism than phagocytosis by alveolar macrophages. Therefore, if particles were to be mainly deposited centrally in the absence of gravity, their residence time in the lungs would be reduced. Conversely, if particles were to be deposited more peripherally in μG than in normal gravity, then they would not be readily cleared by the mucociliary clearance system, thus increasing their residence time in the lungs.
In a recent study, Darquenne and colleagues measured the distribution of regional deposition and retention of coarse radiolabeled particles [99mTc-labeled sulfur colloid, 5-μm mass median aerodynamic diameter (MMAD)] using planar gamma scintigraphy.(12) Particles were inhaled over multiple periods of μG during parabolic flights and in 1G. By collecting data immediately post inhalation and up to 22 hr post deposition, they determined the retention and distribution of deposited particles in the large, intermediate, and small airways as well as in the alveolar region (Fig. 6). Clearance from the large airways was estimated as in previous studies by measuring retentions between 1 and 2 hr post deposition, whereas “24-hr retention” measurements have usually been collected 20–24 hr post deposition.(40–42) The additional time point at 4 hr allowed further distinguishing between intermediate and small airways (Fig. 6). Relative alveolar deposition, as measured by retention at 22 hr, was significantly higher in 1G than in μG (Fig. 6A), most likely because of significant deposition by gravitational sedimentation in the lung periphery when particles were inhaled in 1G. Also, the majority of particles that deposited in 1G did so in the alveolar region, whereas particles that deposited in μG were predominantly located in the larger airways where inertial impaction is expected to be the most efficient (Fig. 6B). These data strongly suggest that for coarse particles (5-μm MMAD) in low gravity, most of the deposited particles are located in the airways where they are readily cleared by the lungs, minimizing any potential toxicological effect they might have.
FIG. 6.
Effect of gravity on retention and distribution of deposited particles. (A) Retention of 5-μm MMAD radiolabeled particles deposited in the right lung as a function of time post inhalation (means±SD, N=5) in μG (open circles) and 1G (closed circles). (B) Relative distribution of deposited particles between the large, intermediate, and small airways, and the alveolar region. *Significantly different from data in 1G, p<0.05 (A) and p<0.001 (B). From Darquenne et al.(12)
Because this study used only coarse particles, the question of the site of deposition of fine particles (i.e., 0.5–2-μm-diameter particles) in a reduced gravity environment remains unanswered. Although there are no human data available to date, some insight may be gained from recent data in animals that suggest that, unlike in 1G, most particles that deposited in μG did so in the lung periphery. Darquenne and colleagues(43) delivered aerosolized 0.9-μm-diameter particles to spontaneously breathing rats in both μG and 1G and measured aerosol deposition in these animals using postmortem magnetic resonance imaging techniques.(44) Comparing deposition in the central (C) region of the lung to that in the peripheral (P) region through the C/P ratio, they showed a reduced C/P ratio in μG compatible with an increase in the relative contribution of peripheral deposition to overall deposition. Even though there are major differences between rats and humans in terms of both airway tree structure(45,46) and ventilation distribution between dependent and nondependent regions of the lungs,(47,48) these animal data support the concept of an increased relative deposition of fine particles (i.e., 0.9 μm) in the lung periphery in the absence of gravity, in agreement with the indirect assessment from aerosol bolus tests described above (Fig. 5). Modeling studies have suggested that the expanding and contracting nature of the alveolar cavities combined with flow irreversibility in the lung periphery causes a non-negligible number of particles to be trapped within the alveolar spaces, where they eventually deposit even in the absence of gravity due to Brownian motion and/or the mechanism of geometrical interception (i.e., a particle is deposited when the distance between the particle center and the alveolar wall is less than the particle's radius).(49,50)
Summary
Studies of aerosol deposition in altered gravity have mostly been performed in humans during parabolic flights both in reduced gravity (μG and lunar gravity) and in hypergravity (∼1.6G). These studies showed a significant effect of gravity on the amount and sites of aerosol deposition in the human lung, with gravity having the largest effect in the lung periphery for particles in the size range 0.5–3 μm (as measured by aerosol bolus inhalations). The relative distribution of deposited particles between gravity levels is also largely affected by particle size. For 5-μm particles, there was a significant shift in distribution of deposited particles away from the lung periphery toward large airways when particles were inhaled in μG. This is the direct result of a decrease in peripheral deposition in the absence of gravity rather than an increase in central deposition. For fine particles (0.5–1 μm), aerosol bolus inhalations and studies in small animals suggest that particles deposit more peripherally in μG than in 1G, beyond the reach of the mucociliary clearance system. Finally, studies approximating conditions of the proposed lunar habitat showed that reduced gravity rather than reduced gas density is the major factor affecting deposition in the lungs of astronauts exposed to airborne particulates.
Acknowledgments
This work was partially supported by grant ES011177 from the National Institute of Environmental Health Sciences (NIEHS) at the National Institutes of Health, by grant NAGW-4372 from NASA, and by the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9-58.
Author Disclosure Statement
The author declares that there are no conflicts of interest.
References
- 1.Prisk GK: Invited review: Microgravity and the lung. J Appl Physiol. 2000;89:385–396 [DOI] [PubMed] [Google Scholar]
- 2.Beeckmans JM: Alveolar deposition of aerosols on the moon and in outer space. Nature. 1966;211:2084960952 [Google Scholar]
- 3.Muir DCF: Influence of gravitational changes on the deposition of aerosols in the lungs of man. Aerosp Med. 1967;38:159–161 [PubMed] [Google Scholar]
- 4.Hoffman RA, and Billingham J: Effect of altered G levels on deposition of particulates in the human respiratory tract. J Appl Physiol. 1975;38:955–960 [DOI] [PubMed] [Google Scholar]
- 5.Darquenne C, Paiva M, and Prisk GK: Effect of gravity on aerosol dispersion and deposition in the human lung after periods of breath-holding. J Appl Physiol. 2000;89:1787–1792 [DOI] [PubMed] [Google Scholar]
- 6.Darquenne C, Paiva M, West JB, and Prisk GK: Effect of microgravity and hypergravity on deposition of 0.5- to 3-μm-diameter aerosol in the human lung. J Appl Physiol. 1997;83:2029–2036 [DOI] [PubMed] [Google Scholar]
- 7.Darquenne C, and Prisk GK: Effect of small flow reversals on aerosol mixing in the alveolar region of the human lung. J Appl Physiol. 2004;97:2083–2089 [DOI] [PubMed] [Google Scholar]
- 8.Darquenne C, and Prisk GK: Aerosol deposition in the human respiratory tract breathing air and 80:20 heliox. J Aerosol Med. 2004;17:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darquenne C, and Prisk GK: Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. Eur J Appl Physiol. 2008;103:687–695 [DOI] [PubMed] [Google Scholar]
- 10.Darquenne C, West JB, and Prisk GK: Deposition and dispersion of 1 μm aerosol boluses in the human lung: effect of micro- and hypergravity. J Appl Physiol. 1998;85:1252–1259 [DOI] [PubMed] [Google Scholar]
- 11.Darquenne C, West JB, and Prisk GK: Dispersion of 0.5–2 μm aerosol in micro- and hypergravity as a probe of convective inhomogeneity in the human lung. J Appl Physiol. 1999;86:1402–1409 [DOI] [PubMed] [Google Scholar]
- 12.Darquenne C, Zeman K, Sa RC, Cooper TK, Fine JM, Bennett WD, and Prisk GK: Removal of sedimentation decreases relative deposition of coarse particles in the lung periphery. J Appl Physiol. 2013;115:546–555 [DOI] [PubMed] [Google Scholar]
- 13.Prisk GK, Sá RC, and Darquenne C: Cardiogenic mixing increases aerosol deposition in the human lung in the absence of gravity. Acta Astronaut. 2013;92:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darquenne C, and Prisk GK: Particulate deposition in the human lung under lunar habitat conditions. Aviat Space Environ Med. 2013;84:190–195 [DOI] [PubMed] [Google Scholar]
- 15.Darquenne C: Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darquenne C, and Paiva M: One-dimensional simulation of aerosol transport and deposition in the human lung. J Appl Physiol. 1994;77:2889–2898 [DOI] [PubMed] [Google Scholar]
- 17.Darquenne C: A realistic two-dimensional model of aerosol transport and deposition in the alveolar zone of the human lung. J Aerosol Sci. 2001;32:1161–1174 [Google Scholar]
- 18.Darquenne C: Heterogeneity of aerosol deposition in a two-dimensional model of human alveolated ducts. J Aerosol Sci. 2002;33:1261–1278 [Google Scholar]
- 19.Heyder J, Blanchard JD, Feldman HA, and Brain JD: Convective mixing in human respiratory tract: estimates with aerosol boli. J Appl Physiol. 1988;64:1273–1278 [DOI] [PubMed] [Google Scholar]
- 20.Darquenne C, Brand P, Heyder J, and Paiva M: Aerosol dispersion in human lung: comparison between numerical simulations and experiments for bolus tests. J Appl Physiol. 1997;83:966–974 [DOI] [PubMed] [Google Scholar]
- 21.Anderson PJ, Blanchard JD, Brain JD, Feldman HA, McNamara JJ, and Heyder J: Effect of cystic fibrosis on inhaled aerosol boluses. Am Rev Respir Dis. 1989;140:1317–1324 [DOI] [PubMed] [Google Scholar]
- 22.Anderson PJ, Hardy KG, Gann LP, Cole R, and Hiller FC: Detection of small airway dysfunction in asymptomatic smokers using aerosol bolus behavior. Am J Respir Crit Care Med. 1994;150:995–1001 [DOI] [PubMed] [Google Scholar]
- 23.Engel LA, Menkes H, Wood LDH, Utz G, Joubert J, and Macklem PT: Gas mixing during breath holding studied by intrapulmonary gas sampling. J Appl Physiol. 1973;35:9–17 [DOI] [PubMed] [Google Scholar]
- 24.West JB, and Hugh-Jones P: Pulsatile gas flow in bronchi caused by the heart beat. J Appl Physiol. 1961;16:697–702 [DOI] [PubMed] [Google Scholar]
- 25.Fukuchi Y, Cosio M, Kelly S, and Engel LA: Influence of pericardial fluid on cardiogenic gas mixing in the lung. J Appl Physiol. 1977;42:5–12 [DOI] [PubMed] [Google Scholar]
- 26.Scheuch G, and Stahlhofen W: Effect of heart rate on aerosol recovery and dispersion in human conducting airways after periods of breathholding. Exp Lung Res. 1991;17:763–787 [DOI] [PubMed] [Google Scholar]
- 27.Lathers CM, Charles JB, Elton KF, Holt TA, Muckai C, Bennett C, and Bungo MW: Acute hemodynamic responses to weightlessness in humans. J Clin Pharmacol. 1989;29:615–627 [DOI] [PubMed] [Google Scholar]
- 28.Lam CW, James JT, McCluskey R, Cowper S, Balis J, and Muro-Cacho C: Pulmonary toxicity of simulated lunar and Martian dusts in mice: I. Histopathology 7 and 90 days after intratracheal instillation. Inhal Toxicol. 2002;14:901–916 [DOI] [PubMed] [Google Scholar]
- 29.Gaier JR: The effects of lunar dust on EVA systems during the Apollo missions, NASA/TM-2005-213610, 2005
- 30.Graf JC: Lunar soils grain size catalog, NASA-RP-1265, 1993
- 31.Conkin J, and Wessel JH, III: Critique of the equivalent air altitude model. Aviat Space Environ Med. 2008;79:975–982 [DOI] [PubMed] [Google Scholar]
- 32.NASA Exploration Atmospheres Working Group: Recommendations for Exploration Spacecraft Internal Atmospheres: The Final Report of the NASA Exploration Atmospheres Working Group, 2010
- 33.Anderson M, Svartengren M, Philipson K, and Camner P: Deposition in man of particles inhaled in air or helium-oxygen at different flow rates. J Aerosol Med. 1990;3:209–216 [Google Scholar]
- 34.Peterson JB, Prisk GK, and Darquenne C: Aerosol deposition in the human lung periphery is increased by reduced-density gas breathing. J Aerosol Med Pulm Drug Deliv. 2008;21:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svartengren M, Anderson M, Philipson K, and Camner P: Human lung deposition of particles suspended in air or in helium/oxygen mixtures. Exp Lung Res. 1989;15:575–585 [DOI] [PubMed] [Google Scholar]
- 36.Lippmann M, Yeates DB, and Albert RE: Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37:337–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moller W, Haussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, and Heyder J: Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. 2004;97:2200–2206 [DOI] [PubMed] [Google Scholar]
- 38.Scheuch G, Stahlhofen W, and Heyder J: An approach to deposition and clearance measurements in human airways. J Aerosol Med. 1996;9:35–41 [DOI] [PubMed] [Google Scholar]
- 39.Linnarsson D, Carpenter J, Fubini B, Gerde P, Karlsson LL, Loftus DJ, Prisk GK, Staufer U, Tranfield EM, and van Westrenen W: Toxicity of lunar dust. Planet Space Sci. 2012;74:57–71 [Google Scholar]
- 40.Zeman KL, Wu J, and Bennett WD: Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J Aerosol Med Pulm Drug Deliv. 2010;23:363–369 [DOI] [PubMed] [Google Scholar]
- 41.Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, and Peden DB: Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy. 2011;41:1719–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ICRP: Human respiratory tract model for radiological protection: a report of the task group of the International Commission on Radiological Protection. ICRP Publication 66 Annex D (Deposition of inhaled particles). Ann ICRP. 1994;24:231–299 [PubMed] [Google Scholar]
- 43.Darquenne C, Borja MG, Oakes JM, Breen EC, Olfert IM, Scadeng M, and Prisk GK: Central to peripheral deposition ratio of inhaled aerosols is less in microgravity than in normal gravity. Am J Resp Crit Care Med. 2012;185:A5587 [Google Scholar]
- 44.Oakes JM, Scadeng M, Breen EC, Prisk GK, and Darquenne C: Regional distribution of aerosol deposition in rat lungs using magnetic resonance imaging. Ann Biomed Eng. 2013;41:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakes JM, Scadeng M, Breen EC, Marsden AL, and Darquenne C: Rat airway morphometry measured from in situ MRI-based geometric models. J Appl Physiol. 2012;112:1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weibel ER, Sapoval B, and Filoche M: Design of peripheral airways for efficient gas exchange. Respir Physiol Neurobiol. 2005;148:3–21 [DOI] [PubMed] [Google Scholar]
- 47.Galvin I, Drummond GB, and Nirmalan M: Distribution of blood flow and ventilation in the lung: gravity is not the only factor. Br J Anaesth. 2007; 98:420–428 [DOI] [PubMed] [Google Scholar]
- 48.Rooney D, Friese M, Fraser JF, Dunster KR, and Schibler A: Gravity-dependent ventilation distribution in rats measured with electrical impedance tomography. Physiol Meas. 2009;30:1075–1085 [DOI] [PubMed] [Google Scholar]
- 49.Ma B, and Darquenne C: Aerosol deposition characteristics in distal acinar airways under cyclic breathing conditions. J Appl Physiol. 2011;110:1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haber S, Yitzhak D, and Tsuda A: Trajectories and deposition sites of spherical particles moving inside rhythmically expanding alveoli under gravity-free conditions. J Aerosol Med Pulm Drug Deliv. 2010;23:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]