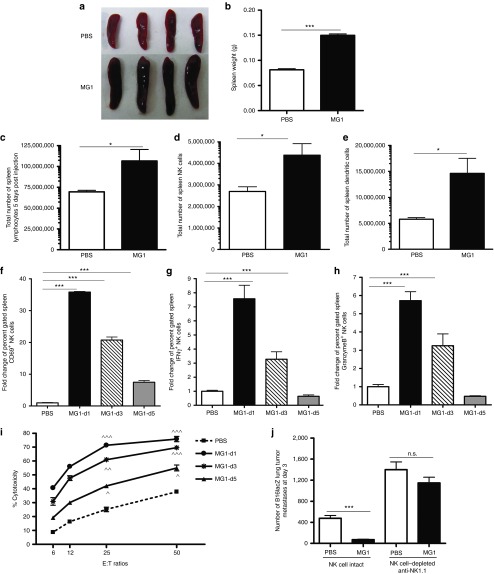
Figure 2.
Activation of NK cell function by MG1 plays a significant role in the reduction of B16lacZ lung metastases. (a–e) B6 mice were treated with MG1 i.v. or PBS and sacrificed at 5 days after virus treatment. Spleens were isolated and assessed as follows: (a) photographs, (b) weight, (c) total number of lymphocytes, (d) total number of NK cells (TCRβ−/CD122+), and (e) total number of DC (CD11c+). (f–h) B6 mice were treated with MG1 (1 × 108 PFU) i.v or PBS and sacrificed at 1, 3, and 5 days after virus treatment. Spleens were harvested to assess NK cell (f) CD69 expression, (g) interferon-γ, (h) Granzyme B secretion, and (i) NK cytotoxicity (^P < 0.05; ^^P < 0.01; ^^^P < 0.001 comparing treatment to PBS controls; the data are displayed as the mean percent (±SD) of chromium release from triplicate wells for the indicated E:T ratios). (j) B6 mice were treated with MG1 i.v. or PBS at day −1. At day 0, all mice received 3 × 105 B16lacZ tumor cell inoculation via tail vein. NK cells were depleted using anti-NK1.1 i.v. at days −4, −1, and +1. Mice were sacrificed at 3 days post–cell injection and the number of lung tumor metastases was quantified. Data are representative of three similar experiments with n = 4–6/group (*P < 0.05; ***P < 0.001, ns, not significant).